Artificial Nervous Systems
- PMID: 40946192
- PMCID: PMC12561383
- DOI: 10.1002/advs.202511478
Artificial Nervous Systems
Abstract
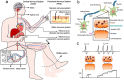
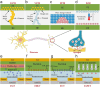
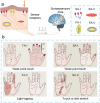
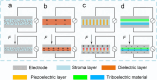
Electronic devices and systems that can emulate the complex cognitive abilities of nervous systems have been an important research topic in recent years. Artificial nervous systems with multimodal perception, neural signal processing, and reflex-driven functionalities have been established by integrating multimodal sensors, neuromorphic synaptic devices, and effector units. The important value of artificial nervous systems is mainly embodied in bioinspired information processing, transmission, and environmental adaptation aspects. However, since this field is still in its early stages, more exploration is needed to realize artificial nervous systems with autonomous adaptability, intelligent feedback, and bio-interfacing applications. This review provides a comprehensive overview of recent advancements, spanning from the fundamental device structure and mechanism of synaptic devices to bio-inspired artificial nervous systems. Finally, a brief perspective of this research field is provided.
Keywords: artificial nervous system; multimodal perception; neural signal processing; neuromorphic device; synaptic device.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


























References
-
- Attwell D., Laughlin S. B., J. Cerebr. Blood Flow Metab. 2001, 21, 1133. - PubMed
-
- Choi S., Yang J., Wang G., Adv. Mater. 2020, 32, 2004659. - PubMed
-
- Zheng Y., Xu H., Li Z., Li L., Yu Y., Jiang P., Shi Y., Zhang J., Huang Y., Luo Q., Adv. Mater. 2025, 37, 2504378. - PubMed
-
- Li L., Xu H., Li Z., Zhong B., Lou Z., Wang L., Adv. Mater. 2025, 37, 2414054. - PubMed
Publication types
MeSH terms
Grants and funding
- 2022YFA1204500/National Key R&D Program of China
- 2022YFA1204504/National Key R&D Program of China
- 2022YFE0198200/National Key R&D Program of China
- T2125005/National Science Fund for Distinguished Young Scholars of China
- BEG124901/Fundamental Research Funds for the Central Universities, Nankai University
LinkOut - more resources
Full Text Sources
