Relieving Chronic Neuropathic Pain With EEG-Neurofeedback
- PMID: 40947911
- PMCID: PMC12434312
- DOI: 10.1111/ene.70363
Relieving Chronic Neuropathic Pain With EEG-Neurofeedback
Abstract
Background: Electroencephalography (EEG)-guided neurofeedback (NFB) is a rarely evaluated neuromodulation technique for the treatment of chronic neuropathic pain.
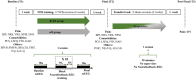
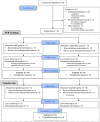
Methods: The analgesic efficacy of EEG-NFB based on two different EEG targets was assessed in two groups of 16 patients with peripheral neuropathic pain. Twelve EEG-NFB sessions were performed over 4 weeks to improve the β1/β2 or α/θ ratio in the EEG signal of the central cortical region contralateral to the pain side. Before and 1 week after treatment, pain interference with daily life (primary endpoint), pain intensity, neuropathic symptom profile, pain catastrophizing, anxiety, depression, fatigue, and sleep quality were evaluated. In addition, EEG was recorded in the resting state before and after EEG-NFB sessions, as well as during the sessions.
Results: Significant analgesic effects were observed on the primary endpoint in both groups. However, β1/β2 training specifically reduced the intensity of ongoing and evoked pain, while anxiety and depression were reduced after α/θ training. Responders to β1/β2 training increased β1 activities or β1/β2 ratio in the resting state or during the EEG-NFB sessions, while an increase in the β1/β2 ratio was also observed during the sessions in responders to α/θ training. In 12 of the 15 responders, a transfer task was performed and showed partial maintenance of therapeutic benefit.
Conclusions: EEG-NFB can produce analgesia with different clinical and neurophysiological impacts according to various EEG targets. These results open perspectives for the use of EEG-NFB protocols in the treatment of chronic neuropathic pain, based on well-defined EEG targets.
Keywords: alpha; beta; chronic pain; theta; treatment.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Radat F., Margot‐Duclot A., and Attal N., “Psychiatric Co‐Morbidities in Patients With Chronic Peripheral Neuropathic Pain: A Multicentre Cohort Study,” European Journal of Pain 17, no. 10 (2013): 1547–1557. - PubMed
-
- Knotkova H., Hamani C., Sivanesan E., et al., “Neuromodulation for Chronic Pain,” Lancet 397, no. 10289 (2021): 2111–2124. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

