Plant Hormone Cytokinin as Aggregation Modulator of Gelsolin Amyloidosis
- PMID: 40947943
- PMCID: PMC12434452
- DOI: 10.1002/psc.70057
Plant Hormone Cytokinin as Aggregation Modulator of Gelsolin Amyloidosis
Abstract
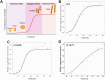
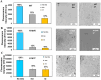
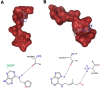
Amyloidosis, a self-assembly of proteins or peptides, is associated with numerous degenerative diseases, such as gelsolin amyloidosis, which remain without a cure. Gelsolin protein is an actin-binding protein, but when aggregated in a diseased state, it is a potential drug target. Specifically, gelsolin mutations, N184K and D187Y, have been linked to renal amyloidosis and systemic progressive deposition of amyloids, respectively. Understanding how such mutations mitigate gelsolin aggregation and how this process can be prevented through small molecule inhibitors is of interest. Herein, we explored the efficacies of plant-based naturally occurring cytokinin (CK) molecules as aggregation modulators in vitro. Using various biophysical methods, such as spectroscopy and microscopy, the aggregation of wild-type gelsolin peptide 184NNGDCFILDL193 and its mutants (N184K, D187Y) was investigated. The mutations significantly promoted aggregation, which is of biological significance. The CK trans-zeatin (tZ) was a more effective disaggregation promoter compared with kinetin (Kin). The experimentally determined IC50 values were in the 9-20 μM range. The mode of inhibition was identified as direct non-covalent complexation between the CK and the peptides by using mass spectrometry and molecular docking studies. Data show that CKs are promising amyloid modulators, which can be easily translatable to other amyloid systems.
Keywords: aggregation; amyloid; cytokinin; gelsolin.
© 2025 The Author(s). Journal of Peptide Science published by European Peptide Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
