Seeding and feeding: nutrition and birth-associated exposures shape gut microbiome assembly in breastfed infants
- PMID: 40948418
- PMCID: PMC12439570
- DOI: 10.1080/19490976.2025.2557981
Seeding and feeding: nutrition and birth-associated exposures shape gut microbiome assembly in breastfed infants
Abstract
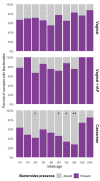
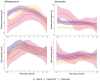
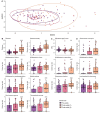
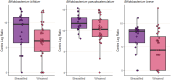
Gut microbiome establishment in early life is influenced by maternal, infant, and environmental factors, with disruptions linked to later disease risk. Although infant diet is a major determinant of microbial composition, longitudinal data in breastfed infants, particularly in the context of birth interventions, remain limited. We profiled 698 stool samples from 84 predominantly or exclusively breastfed infants in the BLOSOM cohort, spanning 10 time points from 1 week to 2 years of age using full-length 16S rRNA gene sequencing and targeted qPCR. After an initial volatile period, microbiome composition and diversity stabilized between months 2 and 5. Introduction of solid foods then triggered a marked ecological shift, with significant increases in diversity (p < 1 × 10-11) and broad compositional restructuring. In contrast, weaning had minimal impact on overall microbiota structure but was associated with lower abundances of several Bifidobacterium species, highlighting the sustained bifidogenic effect of human milk. Cesarean delivery was associated with transient reductions in Bacteroides abundance and prevalence, but did not affect Bifidobacterium, likely due to the bifidogenic effects of human milk. Reductions in Bacteroides, however, were not reflected in quantitative analyses, emphasizing the importance of absolute abundance measures. Our results offer novel insight into gut microbiome development under optimal feeding conditions, suggesting that breastfeeding may buffer early-life microbiome perturbations, while diet transitions exert major and lasting effects.
Keywords: Infant gut microbiome; breastfeeding; delivery mode; infant nutrition; intrapartum antibiotics.
Conflict of interest statement
LFS and DTG receive funding from Medela AG, administered through The University of Western Australia. This funding body had no input into study design, interpretation of results, or decision to publish.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
