Extending the clinical indications of liquid biopsy: plasma next-generation sequencing in suspected lung cancer
- PMID: 40948828
- PMCID: PMC12432645
- DOI: 10.21037/tlcr-2025-443
Extending the clinical indications of liquid biopsy: plasma next-generation sequencing in suspected lung cancer
Keywords: Circulating tumor DNA (ctDNA); cell-free DNA (cfDNA); liquid biopsy; plasma first; suspected lung cancer.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-443/coif). A.R. has received advisory board or speaker bureau honoraria from AstraZeneca, MSD, Novartis, Pfizer, BMS, Takeda, Amgen, Regeneron, Daiichi Sankyo, Merck, and Johnson & Johnson; and has compensated activity for editorial projects from AstraZeneca, MSD, BMS, Novartis, Roche, Regeneron, and Amgen. C.R. reports personal fees for advisory board membership from Novocure; institutional fees for advisory board membership from AstraZeneca, Imagene, MedStar, Amgem, Boehringer-Ingelheim, Hoffmann-La Roche Ltd., Janssen Pharmaceutical, NeoGenomics, Pfizer, Inc. and Regeneron; research collaboration non-remunerated: Guardant, Foundation One; institutional fees as an invited speaker from COR2ED, HPM education IDEOlogy Merck and Roche, OneCell Dx; non-remunerated leadership roles as a scientific board member for the European School of Oncology (ESO), Past Chair on the educational committee for the International Association for Study of Lung Cancer (IASLC), President for the International Society of Liquid Biopsy (ISLB) and Educational Chair for the Oncology Latin American Association (OLA); a remunerated role as Editor-in-Chief for Critical Reviews in Oncology Hematology (CROH); a non-remunerated role as ESMO Faculty Group/Speciality and Faculty Coordinator for metastatic non-small cell lung cancer for European Society for Medical Oncology (ESMO); non-remunerated roles as: Scientific Board Member at ESO (European School of Oncology), external advisor board member of Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica (GENYO), External Advisor of the School of Public Health, University of Granada, Spain, Scientific Committee of the Fondazione Siciliana di Oncologia, and non-remunerated analysis of liquid biopsies in a lung cancer trial for Guardant Health. E.S.S. has received honorarium for consultant work by AstraZeneca, Abbvie, Regeneron, Novartis, Johnson and Johnson, Boehringer-Ingelheim, Novocure, EMD Serono, BMS and Daiichi Sankyo; fees for speaker bureau from Amgen, AstraZeneca, Boehringer-Ingelheim, Biodesix, BMS, Genentech, Guardant Health, Lilly, Pharmacosmos, Jazz Pharmaceuticals, Merck, Novocure, Pfizer, Regeneron, Coherus, Takeda, Johnson and Johnson and Natera; served in the past as chair in IASLC and ASCO committees; and is the actual Vice-President of FLASCO. The authors have no other conflicts of interest to declare.
Figures
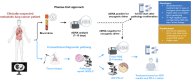
Comment on
-
LIBELULE: A Randomized Phase III Study to Evaluate the Clinical Relevance of Early Liquid Biopsy in Patients With Suspicious Metastatic Lung Cancer.J Thorac Oncol. 2025 Apr;20(4):437-450. doi: 10.1016/j.jtho.2024.12.011. Epub 2024 Dec 16. J Thorac Oncol. 2025. PMID: 39694415 Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
