KLK15 alters connective tissues in hypermobile Ehlers-Danlos syndrome
- PMID: 40949095
- PMCID: PMC12424230
- DOI: 10.1016/j.isci.2025.113343
KLK15 alters connective tissues in hypermobile Ehlers-Danlos syndrome
Abstract
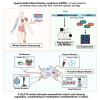
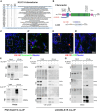
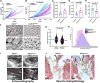
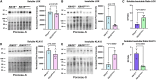
Hypermobile Ehlers-Danlos syndrome (hEDS) is a debilitating multisystem condition characterized by joint hypermobility, chronic pain, and diverse comorbidities, yet its genetic basis remains undefined. Whole-exome sequencing (WES) of 200 patients with hEDS revealed rare and low frequency variants in 14 of 15 kallikrein (KLK) genes, including a recurrent KLK15 missense variant (p.Gly226Asp) segregating in multiple families. KLK15, a secreted serine protease, is expressed in connective and immune tissues and interacts with extracellular matrix (ECM) components, including fibronectin and lysyl oxidase (LOX). A KLK15 knock-in mouse model recapitulated hEDS features in tendons and cardiac valves and exhibited dysregulated cytokine profiles. The variant altered KLK15 and LOX compartmentalization within the ECM, consistent with a dominant-negative effect. These findings identify KLK15 as a contributor to hEDS and reveal broader roles for KLK protease-ECM-immune crosstalk in connective tissue regulation. This study reframes hEDS as a condition involving matrix remodeling and immune signaling beyond collagen defects.
Keywords: Biological process; Body system; Molecular genetics; Non-infectious disease.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

