Mitochondrial Diseases: Molecular Pathogenesis and Therapeutic Advances
- PMID: 40949487
- PMCID: PMC12426487
- DOI: 10.1002/mco2.70385
Mitochondrial Diseases: Molecular Pathogenesis and Therapeutic Advances
Abstract
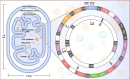
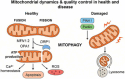
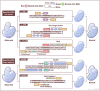
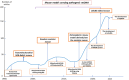
Mitochondrial diseases are a heterogeneous group of inherited disorders caused by pathogenic variants in mitochondrial DNA (mtDNA) or nuclear genes encoding mitochondrial proteins, culminating in defective oxidative phosphorylation and multisystem involvement. Key pathogenic mechanisms include heteroplasmy driven threshold effects, excess reactive oxygen species, disrupted mitochondrial dynamics and mitophagy, abnormal calcium signaling, and compromised mtDNA repair, which together cause tissue-specific energy failure in high demand organs. Recent advances have expanded the therapeutic landscape. Precision mitochondrial genome editing-using mitochondrial zinc finger nucleases, mitochondrial transcription activator-like effector nucleases, DddA-derived cytosine base editor, and other base editing tools-enables targeted correction or rebalancing of mutant genomes, while highlighting challenges of delivery and off-target effects. In parallel, metabolic modulators (e.g., coenzyme Q10, idebenone, EPI-743) aim to restore bioenergetics, and mitochondrial replacement technologies and transplantation are being explored. Despite these promising strategies, major challenges remain, including off-target effects, precise delivery, and ethical considerations. Addressing these issues through multidisciplinary research and clinical translation holds promise for transforming mitochondrial disease management and improving patient outcomes. By bridging the understanding of mitochondrial dysfunction with advanced therapeutic interventions, this review aims to shed light on effective solutions for managing these complex disorders.
Keywords: base editing; gene therapy; genetic medicine; mitochondrial DNA (mtDNA); mitochondrial diseases; mitochondrial gene editing; therapeutic strategies.
© 2025 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Muñoz‐Gómez S. A., Wideman J. G., Roger A. J., and Slamovits C. H., “The Origin of Mitochondrial Cristae From Alphaproteobacteria,” Molecular Biology and Evolution 34, no. 4 (2017): 943–956. - PubMed
-
- Gray M. W., Burger G., and Lang B. F., “Mitochondrial Evolution,” Science (New York, NY) 283, no. 5407 (1999): 1476–1481. - PubMed
-
- Takusagawa M., Misumi O., Nozaki H., et al., “Complete Mitochondrial and Chloroplast DNA Sequences of the Freshwater Green Microalga Medakamo hakoo,” Genes & Genetic Systems 98, no. 6 (2023): 353–360. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
