This is a preprint.
Mice navigate scent trails using predictive policies
- PMID: 40949961
- PMCID: PMC12424787
- DOI: 10.1101/2025.08.27.672631
Mice navigate scent trails using predictive policies
Abstract
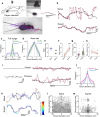
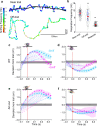
Animals actively sense their environment to extract features of interest to guide behaviors. For mammals, odors are prominent environmental features which are sampled by active modulation of sniffing and orofacial orientation. We sought to understand the strategies that mice use to navigate surface-bound odor cues. We presented mice with dynamic, non-repeating odor trails using a paper treadmill, and observed their behaviors as they collected rewards offered randomly along the trail. By combining high-speed videography over long distances with quantitative behavioral analyses, we find that mice rapidly learn to track odor trails persistently and precisely. Mice with a single nostril blocked can track odor trails, but with a lateral bias and lower precision than control animals. Tracking is severely impaired in animals with both nostrils intact but with interhemispheric communication disrupted by anterior commissure transection. Respiration measurements revealed that a sniff close to the trail triggers a rapid turn towards the trail, a reaction that is lost in commissure-cut animals. Importantly, trail tracking is not simply reactive but involves adaptation to and retention of a short-term memory of the trail geometry and statistics. Our results, recapitulated by a Bayesian inference model, indicate that mice combine immediate sensory information with an internal model of the odor environment to follow odor trails efficiently.
Figures



References
-
- Arulampalam MS, Maskell S, Gordon N, Clapp T. 2002. A Tutorial on Particle Filters for Online Nonlinear/Non-Gaussian Bayesian Tracking. IEEE TRANSACTIONS ON SIGNAL PROCESSING 50.
-
- Barlow HB. 1961. Possible principles underlying the transformations of sensory messages In: Rosenblith WA, editor. Sensory Communication. Cambridge, MA: MIT Press. pp. 217–234.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
