This is a preprint.
INSPECT-SR: a tool for assessing trustworthiness of randomised controlled trials
- PMID: 40950444
- PMCID: PMC12424918
- DOI: 10.1101/2025.09.03.25334905
INSPECT-SR: a tool for assessing trustworthiness of randomised controlled trials
Abstract
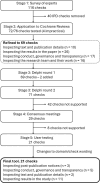
The integrity of evidence synthesis is threatened by problematic randomised controlled trials (RCTs). These are RCTs where there are serious concerns about the trustworthiness of the data or findings. This could be due to research misconduct, including fraud, or due to honest critical errors. If these RCTs are not detected, they may be inadvertently included in systematic reviews and guidelines, potentially distorting their results. To address this problem, the INSPECT-SR (INveStigating ProblEmatic Clinical Trials in Systematic Reviews) tool has been developed to assess the trustworthiness of RCTs. This will allow problematic RCTs to be identified and excluded from systematic reviews. This paper describes the development of INSPECT-SR. The tool and an associated guidance document are presented.
Conflict of interest statement
Declarations JW, CH, GAA, LB, JJK declare funding from NIHR (NIHR203568) in relation to the current project. JW additionally declares Stats or Methodological Editor roles for BJOG, Fertility and Sterility, Reproduction and Fertility, Journal of Hypertension, and for Cochrane Gynaecology and Fertility. AL is on the editorial board of BMC Medical Ethics. TLi declares funding from the National Eye Institute, National Institutes of Health (UG1 EY020522). TLi serves as a methodological editor for Annals of Internal Medicine, the review editor for JAMA Ophthalmology, a Co-Editor-in-Chief for Trials, and is on the editorial board for Journal of Clinical Epidemiology. WL declares funding from the Australian National Health and Medical Research Council, Medical Research Future Fund, and Ramaciotti Foundations. WL serves as an associate editor for Human Reproduction and methodological reviewer for Fertility & Sterility and F&S Reviews. SL declares funding from the University of Melbourne, Norman Beischer Medical Research Foundation, and National Health and Medical Research Council. SL is co-ordinating editor of Cochrane Gynaecology and Fertility and an editor for Human Reproduction Open, Fertility and Sterility, and Trials. FN received funding from the French National Research Agency, the French ministry of health and the French ministry of research. He is a work package leader in the OSIRIS project (Open Science to Increase Reproducibility in Science, funded by the European Union’s Horizon Europe research and innovation programme under grant agreement No. 101094725). FN is also work package leader for the doctoral network MSCA-DN SHARE-CTD (HORIZON-MSCA-2022-DN-01 101120360), funded by the European Union. TJL is an employee of The Cochrane Collaboration and interim Editor in Chief of the Cochrane Library. TA is an employee of The Cochrane Collaboration. EF is an employee of the Cochrane Collaboration and editorial board member of Cochrane Evidence Synthesis and Methods. RR is an employee of The Cochrane Collaboration and editor of Cochrane Evidence Synthesis and Methods. AA and CJV declares funding from the UKRI Metascience Unit (ESRC and Open Philanthropy) for the INSPECT-AI project. UKRI1083: Evaluating the Development and Impact of AI-Assisted Integrity Assessment of Randomised Trials in Evidence Syntheses. ES is a Senior Editor of the Cochrane Database of Systematic Reviews. LP is on the editorial board for Journal of Clinical Epidemiology. SG is an employee of the Cochrane Collaboration. SW is editor for Cochrane Anesthesia and involved in development of the Research Integrity Assessment (RIA) tool for RCTs included in evidence synthesis. ALS is on the Editorial Board for Cochrane Evidence Synthesis Methods, Statistical Editor for Cochrane Neonatal and Co-Convenor for the Cochrane Prospective Meta-Analysis Methods Group. KEH is Associate Convenor for the Cochrane Prospective Meta-Analysis Methods Group. BWM reports consultancy, travel support and research funding from Merck and consultancy for Ferring, Organon, UNILAB and Norgine. RW declares funding from the Australian National Health and Medical Research Council. RW is a Deputy Editor of Human Reproduction Update, an Editorial Board Member of BJOG and Cochrane Gynaecology and Fertility Group, and a former Deputy Editor of Human Reproduction. NOC Between 2020 and 2023 NOC was Coordinating Editor of the Cochrane Pain, Palliative and Supportive Care group, whose activities were funded by The UK National Institute of Health and Care Research (NIHR). He is the Chair of the International Association for the Study of Pain (IASP) Methodology, Evidence Synthesis and Implementation Special Interest Group and has held a grant from the ERA-NET Neuron Co-Fund. LJ is the creator of the scrutiny package in R. VB is the Editor in Chief of the Medical Journal of Australia and on the Editorial Board of Research Integrity and Peer Review. MC declares Coordinating Editor or Editor in Chief roles for James Lind Library, Cochrane Methodology Review Group and Journal of Evidence-Based Medicine, and many years of funded research using randomised trials and systematic reviews. ZA declares membership of the Cochrane Library Editorial Board, and PI on a grant from Children Investment Foundation Fund to University of Liverpool to investigate research integrity of clinical trials related to nutritional supplements in pregnancy. JDe is a member of the Cochrane Emergency and Acute Care Thematic Group. MvW is sign-off Editor for Cochrane, Senior Editor of Cochrane Gynaecology and Fertility and Cochrane Sexually Transmitted Infections, and Editor in Chief of Human Reproduction Update. JAJH declares funding from Open Philanthropy and consultancy for the Gates Foundation and Helena Foundation. HF declares an editorial role at The Lancet. All other authors declare no conflict of interest.
Figures
References
-
- Cochrane. Cochrane Policy for managing potentially problematic studies. Cochrane Database of Systematic Reviews: editorial policies Cochrane Library [Available from: https://www.cochranelibrary.com/cdsr/editorial-policies.
-
- Ker K, Shakur H, Roberts I. Does tranexamic acid prevent postpartum haemorrhage? A systematic review of randomised controlled trials. BJOG. 2016;123(11):1745–52. - PubMed
-
- Carlisle JB. False individual patient data and zombie randomised controlled trials submitted to Anaesthesia. Anaesthesia. 2021;76(4):472–9. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials