This is a preprint.
Autoantibodies neutralizing type I IFNs in 40% of patients with WNV encephalitis in seven new cohorts
- PMID: 40950468
- PMCID: PMC12424919
- DOI: 10.1101/2025.08.31.25334556
Autoantibodies neutralizing type I IFNs in 40% of patients with WNV encephalitis in seven new cohorts
Abstract
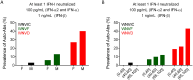
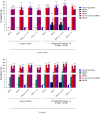
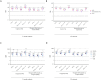
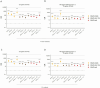
Mosquito-borne West Nile virus (WNV) infection is a growing global health problem. About 0.5% of infected individuals develop encephalitis. We previously showed that 40% of patients in six cohorts had WNV encephalitis because of circulating auto-antibodies (auto-Abs) neutralizing type I IFNs. In seven new cohorts, we found that the prevalence of auto-Abs was highest (40% [17-44%]) in patients with encephalitis, and very low in a small sample of individuals with asymptomatic or mild infection. In the 13 European, Middle-Eastern and American cohorts available, odds ratios for WNV encephalitis in individuals with these auto-Abs relative to those without them in a large sample of the general population untested for WNV infection range from ~20 (OR=17.7; 95% CI: 13.8-22.8, p<10-16) for auto-Abs neutralizing only 100 pg/mL IFN-α2 and/or IFN-ω to >2000 (OR=2218.4; 95% CI: 125.1-39337.7, p<10-16) for auto-Abs neutralizing high concentrations of IFN-α2 and high or low concentrations of IFN-ω. Pre-existing autoantibodies neutralizing type I IFNs are therefore causal for WNV encephalitis in about 40% of patients.
Conflict of interest statement
Declaration of interest J.L.-C. is an inventor on patent application PCT/US2021/042741, filed July 22, 2021, submitted by The Rockefeller University and covering the diagnosis of susceptibility to, and the treatment of, viral disease, and viral vaccines, including COVID-19 and vaccine-associated diseases.
Figures




References
-
- Young J.J., Haussig J.M., Aberle S.W., Pervanidou D., Riccardo F., Sekulić N., Bakonyi T., and Gossner C.M.. Epidemiology of human West Nile virus infections in the European Union and European Union enlargement countries, 2010 to 2018. Euro Surveill. 26:2001095. 2021. doi: 10.2807/1560-7917.ES.2021.26.19.2001095 - DOI - PMC - PubMed
-
- Postler T.S., Beer M., Blitvich B.J., Bukh J., de Lamballerie X., Drexler J.F., Imrie A., Kapoor A., Karganova G.G., Lemey P., Lohmann V., Simmonds P., Smith D.B., Stapleton J.T., and Kuhn J.H.. Renaming of the genus Flavivirus to Orthoflavivirus and extension of binomial species names within the family Flaviviridae. Arch Virol. 168:224. 2023. doi: 10.1007/s00705-023-05835-1 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
