Real-world impact of nirsevimab immunisation against respiratory disease on emergency department attendances and admissions among infants: a multinational retrospective analysis
- PMID: 40950943
- PMCID: PMC12426819
- DOI: 10.1016/j.lanepe.2025.101334
Real-world impact of nirsevimab immunisation against respiratory disease on emergency department attendances and admissions among infants: a multinational retrospective analysis
Abstract
Background: Nirsevimab, a novel monoclonal antibody with a long half-life, has received European Union approval to prevent lower respiratory tract infections (LRTIs) caused by respiratory syncytial virus (RSV) during the first season of exposure. It was implemented in Catalonia (Spain) in the 2023-2024 season. Our main objective was to analyse the impact of the nirsevimab on LRTIs presenting to the Emergency Department (ED) in Catalonia (Spain) by comparing presentations to those at five sites in the United Kingdom (UK) and Rome (Italy).
Methods: In this multi-national retrospective analysis of emergency department attendances and admissions, we retrospectively collected information for all diagnoses, respiratory diagnoses excluding bronchiolitis, and bronchiolitis, for different age groups from 68 hospitals in Catalonia (Spain), one hospital in Rome (Italy), and four hospitals in the UK (Bristol, Leicester, Glasgow, and Edinburgh), from May 1st, 2018, to April 30th 2024. Applying a generalised linear model (GLM) in Poisson regression, we obtained the risk ratio (RR) and 95% confidence intervals (CI) of bronchiolitis in 2023-2024 season compared to the mean of all previous seasons. We analysed data in annual bins, from May 1st to April 30th, excluding 2020-21 as a COVID year, for a total of 5 years of data.
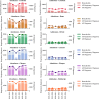
Findings: Data was available for 1,574,392 ED attendances (96,028 for bronchiolitis) and 255,689 hospital admissions (27,691 for bronchiolitis). In the 2023-2024 season, in Catalonia there was a reduction in the RR for bronchiolitis hospital admissions in the youngest infants aged <6 months (0.52, 95% CI: 0.48-0.55). There was also a reduction in Catalonia in the RR for hospital attendances for bronchiolitis in nirsevimab eligible age groups (0-11 months), with a RR of 0.56 (95% CI: 0.54-0.58) for infants <6 m and 0.93 (95% CI: 0.89-0.97) for infants 6-11 m. None of the other sites or age groups showed a significant reduction in the RR for attendances or admissions for the 2023-2024 season compared to previous years.
Interpretation: Nirsevimab had a clear impact in reducing attendances and admissions for infants with bronchiolitis aged <6 months in Catalonia. However, the impact on older infants was less clear.
Funding: None.
Keywords: Bronchiolitis; Children; Lower respiratory tract infections; Nirsevimab.
Crown Copyright © 2025 Published by Elsevier Ltd.
Conflict of interest statement
Dr Damian Roland declares grants or contracts from Wellcome Trust (Funding for administrative support for the BronchStop study), Respiratory Syncytial Virus Consortium in Europe (RESCEU) (Funding to establish the BronchStart/Stop study.), Imperial College London (Funding for administrative support for the BronchStop study), National Institute for Health Research (Funding for data collection for the study). Dr Antoni Soriano-Arandes declares consulting fees, honoraria for lectures and support for attending meetings from Sanofi, MSD and Pfizer, and grants or contracts from la Marato tv3 (Funding addressed to my institution Vall d’Hebron Research Institute for a project about respiratory viruses). Clara Prats declares payments or honoraria for lectures by Societat Catalana de Pediatria (Presentation including results of the impact of nirsevimab in Catalonia), grants or contracts by Fundacion BBVA (Funding paid to the university), and Fundacio la Marato tv3. Aida Perramon-Malavez declares grants or contracts from Fundacio la Marato tv3 (Funding addressed to my institution Vall d’Hebron Research Institute for a project about respiratory viruses). The rest of the authors have nothing to declare.
Figures

References
-
- Beyfortus. European Medicines Agency (EMA) Product information Beyfortus® (nirsevimab) https://www.ema.europa.eu/en/documents/product-information/beyfortus-epa...
-
- Grupo de Trabajo utilización de nirsevimab frente a infección por virus respiratorio sincitial de la Ponencia de Programa y Registro de Vacunaciones. Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad; 2023. https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTr...
LinkOut - more resources
Full Text Sources
Miscellaneous

