Novel Interleukin-11 Inhibitors Attenuate Collagen Production in Patient-Derived Synovial Fibroblasts
- PMID: 40951163
- PMCID: PMC12423567
- DOI: 10.7759/cureus.89850
Novel Interleukin-11 Inhibitors Attenuate Collagen Production in Patient-Derived Synovial Fibroblasts
Abstract
Purpose: Arthrofibrosis (AF), or excessive joint scarring, is a debilitating condition that causes pain and stiffness secondary to osteoarthritis and is often worsened by the surgical trauma of total knee arthroplasty (TKA). The underlying pathology involves dysregulated transforming growth factor-beta 1 (TGF-β1) signaling, which drives fibrogenesis. However, evidence from other organ systems suggests that interleukin-11 (IL-11) mediates fibrosis downstream of TGF-β1. This study examines the relationship between IL-11 and synovial fibrosis, a hallmark of AF, in patients with end-stage knee osteoarthritis (kOA) and evaluates the potential of novel small-molecule IL-11 inhibitors (NMX compounds) as a therapeutic option.
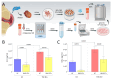
Methods: Synovial fluid and tissue were collected from 24 patients undergoing TKA for kOA, who were divided into low (n = 12) and high (n = 12) fibrosis severity groups. Enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN) and quantitative immunodetection were used to analyze IL-11 and TGF-β1 levels in synovial fluid and to relate them to histological fibrosis status. The effectiveness of NMX compounds (NM922, NM1157, and NM1332) was then tested on commercial synoviocytes and patient-derived fibroblastic synovial cells (FSCs) by measuring IL-11 and collagen type 1 (COL1) production before and during TGF-β1 stimulation in vitro. Parametric tests were employed to compare groups and evaluate associations.
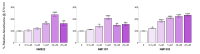
Results: IL-11 and TGF-β1 levels in patient-derived synovial fluid were strongly correlated (R = 0.602; p = 0.003), and each was significantly linked to the severity of synovial fibrosis (R = 0.759; p = 0.0097 and R = 0.527; p < 0.0001, respectively). In vitro, NMX compound treatment reduced TGF-β1-induced IL-11 and COL1 by over 40% compared to untreated controls. Notably, patient-derived FSCs conserved a hyper-fibrotic phenotype, with baseline production of IL-11 and COL1 elevated by 182.86% and 139.63%, respectively, compared to commercial synoviocytes. In the pathologically primed FSCs, the lead compound NM1157 effectively countered fibrogenic stimulation, significantly reducing COL1 production by 44.5% (p = 0.0189) and IL-11 secretion by 28.4% (p = 0.0415).
Conclusions: Our findings demonstrate a strong connection between IL-11 and synovial fibrosis status in patients with advanced kOA, indicating that IL-11 is a key mediator of this process. The ability of the new NMX compounds to significantly reduce fibrotic markers in patient-derived cells offers a promising preclinical basis for developing targeted therapies to prevent or treat AF attributable to kOA or other arthropathies and traumatic joint injuries.
Keywords: interleukin 11; knee arthrofibrosis; osteoarthritis (oa); synovial membrane; transforming growth factor-β1; type 1 collagen.
Copyright © 2025, Schroeder et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Louisiana State University Health Sciences Center, New Orleans, USA issued approval 986. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Dr. Cathy A. Swindlehurst declare(s) non-financial support from Novomedix. Dr. Cathy A. Swindlehurst is the Chief Executive Officer of Novomedix, the company that developed the small-molecule compounds evaluated in this study. The other authors declare no conflicts of interest. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Rates of total joint replacement in the United States: future projections to 2020-2040 using the National Inpatient Sample. Singh JA, Yu S, Chen L, Cleveland JD. J Rheumatol. 2019;46:1134–1140. - PubMed
-
- Arthrofibrosis associated with total knee arthroplasty. Cheuy VA, Foran JR, Paxton RJ, Bade MJ, Zeni JA, Stevens-Lapsley JE. J Arthroplasty. 2017;32:2604–2611. - PubMed
-
- Post-traumatic knee stiffness: surgical techniques. Pujol N, Boisrenoult P, Beaufils P. Orthop Traumatol Surg Res. 2015;101:0–86. - PubMed
-
- Clinical management of arthrofibrosis: state of the art and therapeutic outlook. Ibrahim IO, Nazarian A, Rodriguez EK. JBJS Rev. 2020;8:0. - PubMed
LinkOut - more resources
Full Text Sources
