Therapeutic Vasopressor Use for Postspinal Hypotension in Low-Risk Elective Cesarean Deliveries: A Systematic Review, Network Meta-Analysis, and Trial Sequential Analysis
- PMID: 40951165
- PMCID: PMC12431973
- DOI: 10.7759/cureus.89934
Therapeutic Vasopressor Use for Postspinal Hypotension in Low-Risk Elective Cesarean Deliveries: A Systematic Review, Network Meta-Analysis, and Trial Sequential Analysis
Abstract
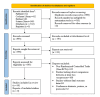
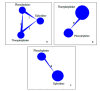
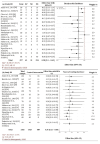
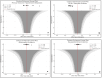
Spinal anesthesia-induced hypotension (SAIH) is a common complication of cesarean delivery (CD), potentially leading to maternal discomfort and fetal compromise. Vasopressors such as norepinephrine (NE), phenylephrine (PE), and ephedrine (EP) are frequently used for treatment, yet their comparative efficacy and safety remain uncertain. This study aimed to assess and compare the effectiveness and tolerability of NE, PE, and EP for managing postspinal hypotension (PSH) in low-risk elective CD. Systematic searches were conducted in PubMed, Embase, Cochrane CENTRAL, ScienceDirect, and ClinicalTrials.gov through June 2025. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD420251074831). We included randomized controlled trials (RCTs) involving parturients undergoing low-risk elective cesarean section who received NE, PE, or EP for the management of PSH. A systematic review, network meta-analysis (NMA), and trial sequential analysis (TSA) were performed. The primary outcome was the successful correction of PSH. Secondary outcomes included maternal bradycardia, nausea, vomiting, neonatal Apgar scores, and umbilical artery pH. Risk of bias was assessed using the Cochrane RoB 2 tool, and the certainty of evidence was graded with the GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology. A total of 16 RCTs encompassing 2,102 parturients were included. NE demonstrated superior efficacy in reversing PSH (odds ratio (OR): 0.23; 95% confidence interval (CI): 0.09-0.58) and was associated with fewer adverse maternal events, including bradycardia (OR: 0.28) and nausea/vomiting (OR: 0.36), compared to PE and EP. Neonatal outcomes were generally comparable across groups, though NE showed a favorable trend in reducing the risk of neonatal acidosis (umbilical artery pH OR: 1.25; 95% CI: 1.06-1.54). Surface under the cumulative ranking curve (SUCRA) rankings and TSA supported the robustness of these findings. NE appears to be the most effective and best-tolerated vasopressor for treating SAIH during elective CD, without compromising neonatal safety. These results support the preferential use of NE over PE and EP in this clinical setting.
Keywords: low-risk elective cesarean delivery; network meta-analysis; norepinephrine; spinal anesthesia; vasopressors.
Copyright © 2025, Babul et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures




References
-
- Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Chooi C, Cox JJ, Lumb RS, et al. http://eng.https Cochrane Database Syst Rev. 2017;8:0. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
