This is a preprint.
OPIOID-EXPRESSING B CELLS SILENCE TUMOR-INFILTRATING NOCICEPTOR NEURONS
- PMID: 40951290
- PMCID: PMC12425075
- DOI: 10.21203/rs.3.rs-7389517/v1
OPIOID-EXPRESSING B CELLS SILENCE TUMOR-INFILTRATING NOCICEPTOR NEURONS
Abstract
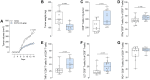
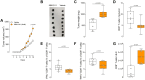
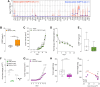
Nociceptor neurons, which transmit pain signals, also regulate immunity by releasing immunomodulatory neuropeptides. In head and neck squamous cell carcinoma (HNSCC) and melanoma, our research has shown that tumor-innervating nociceptors modulate anti-tumor immunity through the release of calcitonin gene-related peptide (CGRP) and its interaction with receptor activity-modifying protein 1 (RAMP1). A retrospective analysis of clinical charts from HNSCC patients revealed that higher pain levels correlated with increased opioid use, perineural invasion, and decreased B-cell infiltration-factors associated with poorer survival outcomes. In silico single-cell RNA sequencing demonstrated that opioid use in HNSCC patients downregulates nociceptin/orphanin FQ (N/OFQ), an endogenous ligand for opioid receptor-like-1 (OPRL1). We identified B cells as the primary source of N/OFQ and observed that high expression of either Pnoc or Oprl1 correlates with better survival in both melanoma and HNSCC. In a mouse model of oral squamous cell carcinoma (oSCC), we found that nociceptor neurons in tongue tumors overexpress Oprl1 and exhibit severe mechanical pain hypersensitivity. Compared to healthy tissue, oSCC tumors have dense infiltration of nociceptor fibers and N/OFQ-expressing B cells. Pharmacological blockade of Oprl1 reduced HNSCC-induced mechanical pain. In a melanoma mouse model, tumor-innervating neurons also overexpressed Oprl1, and similar overexpression was observed when DRG neurons were co-cultured with B16F10 cells. Activating OPRL1 reduced tumor size, enhanced cytotoxic T-cell infiltration, and relieved cancer-induced thermal hypersensitivity. In contrast, depleting CD19+ B cells or blocking OPRL1 led to increased tumor growth, reduced CD8+ T-cell infiltration and cytotoxic potential, exacerbated pain, and elevated CGRP levels. Moreover, we discovered that Ramp1+ B cells express Pnoc, but this expression is suppressed by CGRP. Blocking RAMP1 reduced tumor growth and promoted B-cell Pnoc expression. Overall, these findings suggest that targeting the N/OFQ and RAMP1 pathways could bolster anti-tumor immunity while simultaneously alleviating cancer-induced pain.
Keywords: B Cells; Cancer Neuroscience; Head and Neck Cancer; Melanoma; Neuro-Immunology; Nociceptin; Pain.
Conflict of interest statement
DECLARATIONS OF COMPETING INTEREST. The authors declare no conflicts of interest.
Figures




References
-
- Darragh L. B. et al. Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs. Med (New York, N.Y.) 5, 254–270.e8 (2024).
-
- Zhang Y. et al. Cancer cells co-opt nociceptive nerves to thrive in nutrient-poor environments and upon nutrient-starvation therapies. Cell Metab. 34, 1999–2017.e10 (2022). - PubMed
-
- McIlvried L. A., Atherton M. A., Horan N. L., Goch T. N. & Scheff N. N. Sensory Neurotransmitter Calcitonin Gene-Related Peptide Modulates Tumor Growth and Lymphocyte Infiltration in Oral Squamous Cell Carcinoma. Adv. Biol. 6, e2200019 (2022).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

