Pregnancy-Related Disease Outcomes in Women With Moderate to Severe Multiple Sclerosis Disability
- PMID: 40952740
- PMCID: PMC12439057
- DOI: 10.1001/jamanetworkopen.2025.31581
Pregnancy-Related Disease Outcomes in Women With Moderate to Severe Multiple Sclerosis Disability
Abstract
Importance: Understanding the association between pregnancy and clinical outcomes in women with moderate to severe multiple sclerosis (MS) disability is crucial for guiding family planning and management strategies.
Objective: To assess peripregnancy relapse activity and disability progression in women with a preconception Expanded Disability Status Scale (EDSS) score of 3 or higher.
Design, setting, and participants: This multicenter retrospective cohort study used data from the MSBase Registry, with clinical observations spanning 1984 through 2024. Study cohorts included pregnant women with MS with a preconception EDSS score of 3 or higher (range: 3-10, with higher scores indicating more severe MS-related disability) and propensity score-matched nonpregnant women with MS (controls).
Main outcomes and measures: The main outcomes were peripregnancy annualized relapse rates (ARRs) and time to 6-month confirmed disability worsening (CDW).
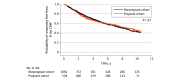
Results: A total of 1631 women with MS were included, of whom 575 were in the pregnant cohort (median [IQR] age at pregnancy, 32.5 [29.1-36.1] years) and 1056 were in the nonpregnant cohort (median [IQR] age, 32.6 [27.5-37.2] years). The median (range) preconception EDSS score was 3.5 (3.0-7.5). Relapse activity decreased during pregnancy, with a 75% reduction in ARR during the first trimester (rate ratio [RR], 0.25; 95% CI, 0.15-0.43), and increased to 36% above preconception levels in the first 3 months post partum (RR, 1.36; 95% CI, 1.06-1.75). Relapse during pregnancy was associated with a higher preconception ARR (odds ratio [OR], 1.56; 95% CI, 1.10-2.20) and preconception use of natalizumab (OR, 4.42; 95% CI, 1.24-23.57) or fingolimod (OR, 14.07; 95% CI, 2.81-91.30). Older age (OR, 0.92; 95% CI, 0.85-0.99) and continuation of disease-modifying therapy into pregnancy (OR, 0.42; 95% CI, 0.19-1.00) were associated with reduced risk. Disease-modifying therapy reinitiation within 1 month post partum was associated with lower odds of early postpartum relapse (OR, 0.45; 95% CI, 0.23-0.86). There was no significant difference in time to CDW between the pregnant and nonpregnant groups (hazard ratio [HR], 1.15; 95% CI, 0.96-1.38). However, ARR during pregnancy (HR, 1.37; 95% CI, 1.13-1.65) and postpartum EDSS score higher than 4 (HR, 2.69; 95% CI, 1.80-4.03) were associated with shorter time to CDW.
Conclusions and relevance: In this cohort study, women with moderate to severe MS disability exhibited a pattern of peripregnancy relapse activity similar to that reported in women with less disability. Pregnancy was not associated with worse long-term disability outcomes, although optimizing disease control in the peripregnancy period remained critical.
Conflict of interest statement
Figures


References
-
- The Multiple Sclerosis International Federation . Atlas of MS, 3rd edition: Part 1—Mapping Multiple Sclerosis Around the World. Multiple Sclerosis International Federation; 2020. Accessed December 10, 2024. https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidem...
-
- De Las Heras V, De Andrés C, Téllez N, Tintoré M; EMPATIE Study Group . Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult Scler. 2007;13(8):981-984. doi: 10.1177/1352458507077896 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

