Five-Year Outcomes from Deep Brain Stimulation of the Subthalamic Nucleus for Parkinson Disease
- PMID: 40952750
- PMCID: PMC12439180
- DOI: 10.1001/jamaneurol.2025.3373
Five-Year Outcomes from Deep Brain Stimulation of the Subthalamic Nucleus for Parkinson Disease
Abstract
Importance: The Implantable Neurostimulator for the Treatment of Parkinson's Disease (INTREPID) trial was a randomized, double-blind, sham-controlled study of subthalamic nucleus (STN) deep brain stimulation (DBS) for the treatment of Parkinson disease (PD).
Objective: To evaluate the long-term (5-year) outcomes and safety of STN-DBS for PD.
Design, setting, and participants: This was a prospective, randomized (3:1), 12-week double-blind sham-controlled study at 23 movement disorder centers across the US with an open-label 5-year follow-up. Patients were implanted and followed up with the Vercise DBS system from May 2013 to December 2022. Eligibility required diagnosis of bilateral idiopathic PD with more than 5 years of motor symptoms, more than 6 hours per day of poor motor function, modified Hoehn and Yahr Scale scores higher than 2, Unified Parkinson's Disease Rating Scale (UPDRS-III) score of 30 or higher (medication-off state), and 33% or higher improvement in UPDRS-III medication-on score.
Intervention: Bilateral STN-DBS for moderate to advanced PD.
Main outcomes and measures: Primary outcomes included changes in UPDRS and dyskinesia scores, quality-of-life measures, and safety assessments. Exploratory analyses included medication reduction and DBS association with motor signs.
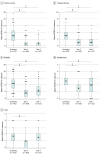
Results: A total of 313 patients were enrolled with 191 receiving the DBS system, and 137 participants (72%) completed the study. The study population had a mean (SD) age of 60 (7.9) years, with 139 (73%) male participants. Motor function without medication as measured by UPDRS-III improved from a mean (SD) of 42.8 (9.4) to 21.1 (10.6) at year 1 (51%; 95% CI, 49%-53%; P < .001) and 27.6 (11.6) at year 5 (36%; 95% CI, 33%-38%; P < .001). Activities of daily living without medication as measured by UPDRS-II improved from a mean (SD) of 20.6 (6.0) to 12.4 (6.1) at year 1 (41%; 95% CI, 38%-42%; P < .001) and 16.4 (6.5) at year 5 (22%; 95% CI, 18%-23%; P < .001). Dyskinesia scores decreased from 4.0 (5.1) to 1.0 (2.1) at year 1 (75%; 95% CI, 73%-75%; P < .001) and to 1.2 (2.1) at year 5 (70%; 95% CI, 63%-75%; P < .001). The levodopa equivalent dose was reduced by 28% at year 1, remaining stable at year 5 (28%; 95% CI, 26%-31%; P < .001). The most common serious adverse event was infection (9 participants). Ten deaths were reported, none related to the study.
Conclusions and relevance: Although STN-DBS outcomes declined slightly, possibly due to the progressive nature of the disease, patients with PD sustained significant improvement in motor and activities of daily living scores, along with a stable reduction in anti-parkinsonian medication over the 5-year follow-up period.
Conflict of interest statement
Figures

References
-
- Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020;19(6):491-501. doi: 10.1016/S1474-4422(20)30108-3 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

