Fast Acute Sedation at Intensive Care vs. High-Dose IV Anti-seizure Medication for Treatment of Non-convulsive Status Epilepticus: A Randomized, Multicenter Trial
- PMID: 40953286
- PMCID: PMC12440533
- DOI: 10.1097/CCE.0000000000001311
Fast Acute Sedation at Intensive Care vs. High-Dose IV Anti-seizure Medication for Treatment of Non-convulsive Status Epilepticus: A Randomized, Multicenter Trial
Abstract
Background: The management of refractory status epilepticus (SE) remains an area of low evidence with varying management strategies. Treatment in the ICU is often postponed due to potential complications from sedation, and it is unknown if its efficacy is superior to additional treatment attempts with IV anti-seizure medications (ASMs). The Fast Acute Sedation at Intensive Care vs. High-Dose IV Anti-Seizure Medication for Treatment of Non-Convulsive Status Epilepticus (FAST) trial aims to compare the efficacy of rapid sedation in the ICU vs. add-on high-dose IV ASM alone for the treatment of refractory SE.
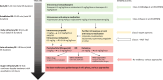
Methods/results: This prospective, randomized, multicenter trial will enroll adult patients with non-convulsive status epilepticus (NCSE) who either meet current EEG criteria or have unambiguous NCSE with minor motor phenomena ("subtle SE") but without ongoing tonic-clonic seizures that are refractory to benzodiazepines and treatment with at least one second-line ASM. Patients will be randomized to receive either rapid deep sedation for 20 hours with propofol and eventually low-dose midazolam or additional high-dose IV anticonvulsant therapy (levetiracetam, valproate, fosphenytoin, lacosamide, or topiramate) in the intermediate care unit. The primary endpoint is treatment failure, either defined as NCSE on EEG 24 hours after randomization or persistent NCSE after 3 hours despite therapy on continuous EEG or clinically. Secondary endpoints include assessment of new-onset neurologic deficits and modified Rankin Scale at discharge, economic analyses, length of hospital stay, in-hospital infections, and survival. Evaluations will be performed at baseline, discharge, and 3, 6, 12, and 24 months. The target sample size is 116 patients; we expect to have to randomize about 140 patients to reach the required number of patients.
Conclusions: The FAST trial is the first randomized clinical trial to investigate refractory NCSE. Regardless of the outcome, the results of this trial protocol will provide new class 1 evidence for the treatment of NCSE and establish the standard of care for this patient population in the future.
Trial registration: EU CT: 2024-515507-18-00/clinicaltrials.gov: NCT05263674.
Copyright © 2025 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Dr. Cornwall reports travel support from Jazz Pharma. Dr. Christensen received honoraria from serving on the Scientific Advisory Board of UCB Nordic and Eisai AB, honoraria from giving lectures from UCB Nordic and Eisai AB, and funding for a trip from UCB Nordic. Dr. Beier reports travel support from Angelini and Jazz Pharma and honoraria for teaching/lecturing from EISAI, UCB, and Angelini. The study was fully funded by the Danish Regions.
Figures

References
-
- Oßwald HM, Harenberg L, Jaschonek H, et al. : Development and validation of the Heidelberg Neurological Triage System (HEINTS). J Neurol 2019; 266:2685–2698 - PubMed
-
- Kämppi L, Puolakka T, Ritvanen J, et al. : Burden of suspected epileptic seizures on emergency services: A population-based study. Eur J Neurol 2023; 30:2197–2205 - PubMed
-
- Trinka E, Cock H, Hesdorffer D, et al. : A definition and classification of status epilepticus—report of the ILAE Task force on classification of status epilepticus. Epilepsia 2015; 56:1515–1523 - PubMed
-
- Leitinger M, Gaspard N, Hirsch LJ, et al. : Diagnosing nonconvulsive status epilepticus: Defining electroencephalographic and clinical response to diagnostic intravenous antiseizure medication trials. Epilepsia 2023; 64:2351–2360 - PubMed
-
- Leitinger M, Beniczky S, Rohracher A, et al. : Salzburg consensus criteria for non-convulsive status epilepticus—approach to clinical application. Epilepsy Behav 2015; 49:158–163 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

