Risk of Epilepsy and Factors Associated With Time to Seizure Remission in Anti-LGI1 Encephalitis: Long-Term Outcome in 236 Patients
- PMID: 40953325
- PMCID: PMC12440303
- DOI: 10.1212/NXI.0000000000200469
Risk of Epilepsy and Factors Associated With Time to Seizure Remission in Anti-LGI1 Encephalitis: Long-Term Outcome in 236 Patients
Abstract
Background and objectives: Autoimmune encephalitis (AIE) with anti-leucine-rich glioma-inactivated 1 (LGI1) antibodies typically manifests with subacute cognitive deficits, seizures, and psychiatric symptoms, mostly in older adults. Immunotherapy (IT) leads to the cessation of seizures in most patients, yet some develop AIE-associated epilepsy (AEAE) and persistent cognitive deficits. The aim of this large multicentric retrospective observational cohort study was to assess long-term outcomes of patients with anti-LGI1 encephalitis regarding seizures and AEAE and to identify associated factors.
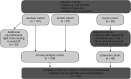
Methods: We included patients with anti-LGI1 encephalitis from 3 national referral centers/consortia meeting the following inclusion criteria: (I) definite LGI1 limbic encephalitis (Graus criteria); (II) occurrence of seizures; and (III) follow-up period ≥24 months. We aimed to (1) determine the risk of seizure recurrence (ROSR) on remission, (2) investigate clinical and paraclinical biomarkers for an effect on time to seizure remission using Cox proportional hazard modeling (n = 188), and (3) assess the risk of AEAE and determine associated factors (n = 236).
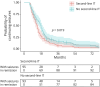
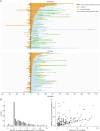
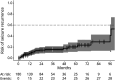
Results: AEAE was observed in 5.9% (16/271) of the full cohort. Both AEAE (16/16 vs 129/215, p = 0.001) and longer time to seizure remission (OR 1.36 per year, p = 0.025) were associated with persistent cognitive impairment. Patients with pilomotor seizures had a lower rate of seizure remission (hazard ratio [HR] 0.58, 95% CI 0.55-0.60, p < 0.001) while patients under IT administration had a higher rate of seizure remission over time (HR 12.4, 95% CI 9.67-16.0, p < 0.001). In addition, patients receiving second-line IT tended to achieve earlier seizure remission (log-rank test, p = 0.019). The ROSR at 12, 60, and 120 months on seizure remission was 9% (95% CI 4.5%-13%), 20% (95% CI 11%-28%), and 53% (95% CI 14%-74%), respectively.
Discussion: In conclusion, our results demonstrate that AEAE in anti-LGI1 encephalitis is rare and suggest that the diagnosis of epilepsy is inappropriate in patients reaching seizure remission because of a relatively low ROSR. Accordingly, on seizure remission, the diagnosis of acute symptomatic seizures would be appropriate. Moreover, we validate and quantify the importance of IT for seizure remission and identify biomarkers associated with lower rates of seizure remission. Late remission of seizures and AEAE were associated with persistent cognitive impairment.
Conflict of interest statement
T. Baumgartner, M. Freyberg, L. Campetella, Y.S. Crijnen, J. Dargvainiene, C. Behning, C.G. Bien, A. Rada, H. Prüss, R. Rößling, S. Kovac, C. Strippel, F.S. Thaler, K. Eisenhut, J. Lewerenz, F. Becker, R. Reinecke, and M. Malter report no disclosures. K-W Sühs received honoraria for lectures or travel reimbursements for attending meetings from Biogen, Merck, Mylan, Roche, Bavarian Nordic, Viatris, and Bristol-Myers Squibb, as well as research support from Bristol-Myers Squibb. S.C. Tauber reports no disclosures. FV Podewils has received personal fees as a speaker or for serving on advisory boards from Angelini, Arvelle, Bial, Desitin Arzneimittel, Eisai, Jazz Pharmaceuticals, UCB Pharma, and Zogenix. N. Melzer, K-P. Wandinger, R.A. M. Fernandez Ceballos, J. Kuhle, K. Berger, T. Bauer, T. Rüber, A. Racz, A.J. Becker, J. Pitsch, G. Kuhlenbäumer, and S. Muñiz-Castrillo report no disclosures. F. Leypoldt discloses speaker honoraria from Grifols, Argenx, and Roche; travel funding from Grifols; and service on advisory boards for Roche and Argenx. MJ Titulaer has filed a patent, on behalf of the Erasmus MC, for methods for typing neurologic disorders and cancer, and devices for use therein, and has received research funds for serving on a scientific advisory board of AmGen and for consultation at Guidepoint Global LLC and UCB, royalties from UpToDate Inc., and an unrestricted research grant from Euroimmun AG and from CSL Behring. R. Surges reports no disclosures. Go to
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
