Progressive Encephalomyelitis With Rigidity and Myoclonus With Glycine Receptor Antibodies: Clinical Features and Outcomes
- PMID: 40953327
- PMCID: PMC12440304
- DOI: 10.1212/NXI.0000000000200473
Progressive Encephalomyelitis With Rigidity and Myoclonus With Glycine Receptor Antibodies: Clinical Features and Outcomes
Abstract
Background and objectives: The aim of this study was to describe the clinical features and long-term outcome of patients with glycine receptor (GlyR) antibody-mediated progressive encephalomyelitis with rigidity and myoclonus (PERM), a disease commonly included under the term of stiff-person spectrum disorders (SPSDs).
Methods: We conducted a retrospective analysis of patients with PERM and GlyR antibodies diagnosed in our laboratory and a systematic literature review (following Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] 2020 reporting guideline) of previously reported patients with sufficient clinical information and ≥12 months of follow-up. Neurologic disability was measured with the modified Rankin Scale (mRS). Relapses were defined as any event occurring >6 months after the first episode that required immunotherapy.
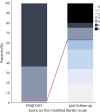
Results: Forty-one patients were identified, 22 from our database and 19 from the literature. The median age was 58 years (IQR: 43-66 years), and 36 (88%) were male and 5 female. The median time from symptom onset to admission was 2 weeks (IQR: 1-4 weeks). Predominant presentations included brainstem symptoms, mainly dysphagia and trismus, in 23 patients (56%); muscle stiffness and myoclonus in 9 (22%); dysesthesias or pruritus in 7 (17%); and cacosmia with dysgeusia in 2 (5%). Five patients (12%) never developed muscle stiffness. The median (range) mRS score at nadir was 5 (3-5). All patients received immunotherapy. Eleven patients died, 8 from complications of PERM. There were 12 relapses in 10 (28%) of 36 patients who lived >6 months. All relapses responded to immunotherapy. The functional status at the last visit, median time 24 months (IQR: 18-72 months), was good (mRS score <3) in 23 (70%) of the 33 patients who did not die from PERM. Age (HR: 1.06; 95% CI 1.01-1.11; p = 0.019) and admission to the intensive care unit (HR: 5.26; 95% CI 1.41-19.57, p = 0.013) were independent predictors of bad outcome (mRS score ≥3).
Discussion: GlyR antibody-mediated PERM is a rapidly progressive and severe disease that predominantly affects men and frequently presents with brainstem involvement. Its distinct demographic and clinical features suggest that it should be considered separately from SPSDs, which typically follows a chronic course and is more commonly associated with glutamic acid decarboxylase antibodies.
Conflict of interest statement
J. Dalmau receives royalties from Athena Diagnostics for the use of Ma2 as an autoantibody test and from Euroimmun for the use of NMDA, GABAB receptor, GABAA receptor, DPPX, and IgLON5 as autoantibody tests and has received an unrestricted research grant from Euroimmun. F. Graus holds a patent licensed to Euroimmun for the use of IgLON5 in an autoantibody test, for which he receives royalties and receives honoraria from MedLink Neurology for his role as associate editor. The other authors report no relevant disclosures. Full disclosure form information provided by the authors is available with the full text of this article at
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials