Cranberry improves metabolic syndrome-related organ dysfunction in rats by modulating AMPK/SREBP1, ROCK1 and TGF-β1
- PMID: 40954151
- PMCID: PMC12436610
- DOI: 10.1038/s41598-025-16925-2
Cranberry improves metabolic syndrome-related organ dysfunction in rats by modulating AMPK/SREBP1, ROCK1 and TGF-β1
Abstract
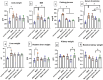
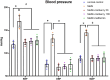
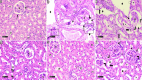
Metabolic syndrome (MetS) is a widespread, complex health issue that poses a substantial global health burden with increased healthcare costs and reduced quality of life, necessitating effective prevention and management strategies. This study aimed to investigate the potential therapeutic effects of cranberry extract (Vaccinium macrocarpon) and metformin on metabolic syndrome in a rat model. Forty rats were divided into the following groups: normal control, MetS (high fat and fructose for 4 weeks followed by streptozotocin 35 mg/kg, i.p.), MetS + cranberry (50 mg/kg), MetS + cranberry (100 mg/kg), and MetS + metformin (200 mg/kg) groups. Treatments were given orally for four weeks with the continuation of a high-fat and high-fructose diet. The evaluations included key metabolic parameters, liver and kidney pathology, and relevant molecular pathways. The present results revealed that MetS induction significantly increased body weight, BMI, fasting glucose, and OGTT results; impaired lipid profile, creatinine and blood pressure; and upregulated hepatic gene expression of Rho-associated protein kinase 1 (Rock1 ) and sterol regulatory element-binding transcription factor 1 (Srebf1), which encodes the protein SREBP-1c. In addition to hepatic and renal structural abnormalities, increased collagen and increased iNOS/TGF-β1 immunoreactivity were observed. Cranberry ameliorated metabolic parameters in a dose-dependent manner, upregulated adenosine monophosphate-activated protein kinase (AMPK), downregulated Rock1 and Srebf1 expression, improved the histopathology of the liver and kidney and decreased the immunoexpression of iNOS and TGF-β1. The results for cranberry were generally comparable to those for metformin. In conclusion, cranberry extract is potentially a safe therapeutic strategy for MetS, offering broad-spectrum action, organ protection, and molecular pathway modulation. These findings strongly support cranberry as a promising natural approach for managing MetS.
Keywords: Rock1; AMPK/Srebf1; Cranberry extract; Metabolic syndrome; TGF-β1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Prior publication: The data presented in this manuscript have not been published previously.
Figures










References
-
- Bruce, K. D. & Hanson, M. A. The developmental origins, mechanisms, and implications of metabolic syndrome. J. Nutr.140 (2010). - PubMed
-
- Xue, L., Liu, C., Ma, H., Seeram, N. P. & Neto, C. C. Anti-inflammatory activities of cranberry fruit extracts in human THP-1 monocytes are influenced by their phytochemical composition. ACS Food Sci. Technol.2, (2022).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

