Methodological validation of Miro1 retention as a candidate Parkinson's disease biomarker
- PMID: 40954161
- PMCID: PMC12436598
- DOI: 10.1038/s41531-025-01115-8
Methodological validation of Miro1 retention as a candidate Parkinson's disease biomarker
Abstract
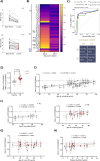
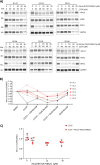
Mitochondrial markers help stratify Parkinson's disease (PD) patients. We use high-throughput blotting to quantify Miro1, Mfn2, and VDAC levels in fibroblasts, blood cells, and iPSC-derived neurons. Miro1 is specifically retained in PD cells but degraded in healthy ones after mitochondrial depolarization. We correlate Miro1 retention scores with pathogenic mutations, genetic background, age, and clinical data. This scalable assay and quantifiable score for mitochondrial-PD support biomarker development and pharmacological screening.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- MacAskill, A. F. et al. GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell Neurosci.40, 301–312 (2009). - PubMed
-
- Guo, X. et al. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron47, 379–393 (2005). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

