Delaying pyroptosis with an AI-screened gasdermin D pore blocker mitigates inflammatory response
- PMID: 40954252
- PMCID: PMC12479363
- DOI: 10.1038/s41590-025-02280-x
Delaying pyroptosis with an AI-screened gasdermin D pore blocker mitigates inflammatory response
Abstract
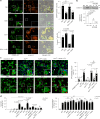
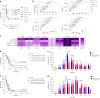
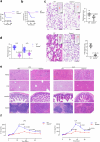
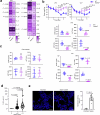
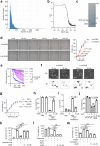
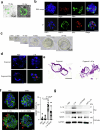
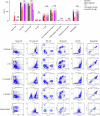
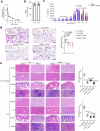
The formation of membrane pores by cleaved N-terminal gasdermin D (GSDMD-NT) results in the release of cytokines and inflammatory cell death, known as pyroptosis. Blocking GSDMD-NT pores is an attractive and promising strategy for mitigating inflammation. Here we demonstrate that SK56, an artificial intelligence-screened peptide, effectively obstructs GSDMD-NT pores and inhibits pyroptosis and cytokine release in macrophages and human peripheral blood leukocyte-induced pyroptosis. SK56 prevents septic death induced by lipopolysaccharide or cecal ligation and puncture surgery in mice. SK56 does not influence cleavage of interleukin-1β or GSDMD. Instead, SK56 inhibits the release of cytokines from pyroptotic macrophages, mitigates the activation of primary mouse dendritic cells triggered by incubation with pyroptotic cytomembranes and prevents widespread cell death of human alveolar organoids in an organoid-macrophage coculture model. SK56 blocks GSDMD-NT pores on lipid-bilayer nanoparticles and enters pyroptotic macrophages to inhibit mitochondrial damage. SK56 presents new therapeutic possibilities for counteracting inflammation, which is implicated in numerous diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
-
- Ding, J. J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature535, 111–116 (2016). - PubMed
-
- Shi, J. J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature526, 660–665 (2015). - PubMed
-
- Heilig, R. et al. The gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur. J. Immunol.48, 584–592 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- 2018DG008, 202101AT070301, 202201AS070070/Yunnan Provincial Science and Technology Department (Yunnan Department of Science and Technology)
- 82222038/National Natural Science Foundation of China (National Science Foundation of China)
- 82360075/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Research Materials

