Influenza vaccine effectiveness and genetic diversity: insights from end-of-season community surveillance, France, 2024-2025
- PMID: 40955037
- PMCID: PMC12517415
- DOI: 10.1080/22221751.2025.2562045
Influenza vaccine effectiveness and genetic diversity: insights from end-of-season community surveillance, France, 2024-2025
Abstract
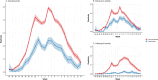
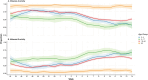
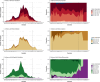
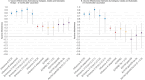
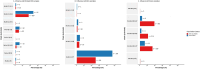
Influenza 2024-2025 season in France was characterized by prolonged duration, unusual co-circulation of all three viruses (A(H1N1)pdm09, A(H3N2), B/Victoria) with several subclades, and substantial healthcare impact. We aimed to investigate the impact of influenza genetic diversity on vaccine effectiveness (VE). A test-negative design study was conducted to estimate VE in a large cohort from the RELAB network of community-based laboratories (n = 77,704 patients). A subset of sequenced samples (n = 2,119 patients) allowed VE estimation for several clades and subclades as well as comparison of subclade distribution by vaccination status. Vaccine coverage was 45% in patients aged 65 years and older (65+). VE based on PCR-confirmed infections was 44% (95% CI: 41-48%) and lower in 65+ individuals, at 25% (95% CI:18-31%) especially for type A virus (23%; 95% CI: 13-32%) compared to type B virus (57%; 95% CI: 35-72%). Sequencing-confirmed VE among individuals vaccinated 15 days to 3 months prior testing, was 41% (95% CI: 14-60%) for A(H1N1)pdm09 and 47% (95% CI: 21-64%) for its main subclade 5a.2a(C.1.9.3); A(H3N2) estimate was 30% (95% CI:5-48%) and 31% (95% CI:4-50%) for 2a.3a.1(J.2) sublclade. The emerging A(H1N1)pdm09 5a.2a.1 (D.3.1) subclade was significantly more frequent among vaccinated individuals compared to unvaccinated. The low vaccine coverage combined with the notably low effectiveness against A(H3N2) and for type A in elderly may have contributed to the high influenza activity this season. The emergence of A(H1N1)pdm09 5a.2a.1 (D.3.1) raises uncertainty and requires surveillance.
Keywords: Influenza; acute respiratory infection; community testing; surveillance; vaccine effectiveness.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- European Centre for Disease Prevention and Control (ECDC) . (2025). WHO Regional Office for Europe. European Respiratory Virus Surveillance Summary (ERVISS). https://erviss.org/.
-
- UK Health Security Agency (UKHSA) . (2025). Influenza, UKHSA data dashboard. https://ukhsa-dashboard.data.gov.uk/respiratory-viruses/influenza.
-
- Istituto Superiore di Sanità . (2025). RespiVirNet – Sorveglianza integrata dei virus respiratori [Integrated Respiratory Virus Surveillance]. https://respivirnet.iss.it/pagine/rapportoInflunet.aspx.
-
- Robert Koch Instute (RKI) . (2025). RKI – Aktueller Wochenbericht – GrippeWeb-Wochenbericht. [Current weekly report – GrippeWeb weekly report]. https://www.rki.de/DE/Themen/Forschung-und-Forschungsdaten/Sentinels-Sur....
-
- Santé publique France (SPF) . (2025). Infections respiratoires aiguës (grippe, bronchiolite, COVID-19) [Acute respiratory infections (influenza, bronchiolitis, COVID-19) 2024-2025]. Bilan de la saison 2024-2025. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
