Structural determinants for red-shifted absorption in higher-plants Photosystem I
- PMID: 40955088
- PMCID: PMC12589708
- DOI: 10.1111/nph.70562
Structural determinants for red-shifted absorption in higher-plants Photosystem I
Abstract
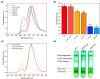
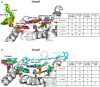
Higher plants Photosystem I absorbs far-red light, enriched under vegetation canopies, through long-wavelength Chls to enhance photon capture. Far-red absorption originates from Chl pairs within the Lhca3 and Lhca4 subunits of the LHCI antenna, known as the 'red cluster', including Chls a603 and a609. We used reverse genetics to produce an Arabidopsis mutant devoid of red-shifted absorption, and we obtained high-resolution cryogenic electron microscopy structures of PSI-LHCI complexes from both wild-type and mutant plants. Computed excitonic coupling values suggested contributions from additional nearby pigment molecules, namely Chl a615 and violaxanthin in the L2 site, to far-red absorption. We investigated the structural determinants of far-red absorption by producing further Arabidopsis transgenic lines and analyzed the spectroscopic effects of mutations targeting these chromophores. The two structures solved were used for quantum mechanics calculations, revealing that excitonic interactions alone cannot explain far-red absorption, while charge transfer states were needed for accurate spectral simulations. Our findings demonstrate that the molecular mechanisms of light-harvesting under shaded conditions rely on very precise tuning of chromophore interactions, whose understanding is crucial for designing light-harvesting complexes with engineered absorption spectra.
Keywords: Lhca; Photosystem I; far‐red; light‐harvesting; low‐energy absorption; photosynthesis; red forms.
© 2025 The Author(s). New Phytologist © 2025 New Phytologist Foundation.
Conflict of interest statement
None declared.
Figures





References
-
- Akhtar P, Lambrev PH. 2020. On the spectral properties and excitation dynamics of long‐wavelength chlorophylls in higher‐plant photosystem I. Biochimica et Biophysica Acta – Bioenergetics 1861: 148274. - PubMed
-
- van Amerongen H, van Grondelle R. 2001. Understanding the energy transfer function of LHCII, the major light‐harvesting complex of green plants. The Journal of Physical Chemistry B 105: 604–617.
-
- Ashenafi EL, Nyman MC, Shelley JT, Mattson NS. 2023. Spectral properties and stability of selected carotenoid and chlorophyll compounds in different solvent systems. Food Chemistry Advances 2: 100178.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources


