Double-positive T cells form heterotypic clusters with circulating tumor cells to foster cancer metastasis
- PMID: 40955669
- PMCID: PMC12435850
- DOI: 10.1172/JCI193521
Double-positive T cells form heterotypic clusters with circulating tumor cells to foster cancer metastasis
Abstract
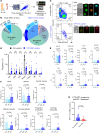
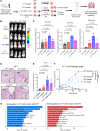
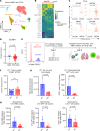
The immune ecosystem is central to maintaining effective defensive responses. However, it remains largely understudied how immune cells in the peripheral blood interact with circulating tumor cells (CTCs) in metastasis. Here, blood analysis of patients with advanced breast cancer revealed that over 75% of CTC-positive blood specimens contained heterotypic CTC clusters with CD45+ white blood cells (WBCs), which correlates with breast cancer subtypes, racial groups, and decreased survival. CTC-WBC clusters included overrepresented T cells and underrepresented neutrophils. Specifically, a rare subset of CD4 and CD8 double-positive T (DPT) cells was 140-fold enriched in CTC clusters versus their frequency in WBCs. DPT cells shared properties with CD4+ and CD8+ T cells but exhibited unique features of T cell exhaustion and immune suppression. Mechanistically, the integrin heterodimer α4β1, also named very late antigen 4 (VLA-4), in DPT cells and its ligand, VCAM1, in tumor cells are essential mediators of DPT-CTC clusters. Neoadjuvant administration of anti-VLA-4 neutralizing antibodies markedly blocked CTC-DPT clusters, inhibited metastasis, and extended mouse survival. These findings highlight a pivotal role of rare DPT cells in fostering cancer dissemination through CTC clustering. It lays a foundation for developing innovative biomarker-guided therapeutic strategies to prevent and target cancer metastasis.
Keywords: Breast cancer; Clinical Research; Diagnostics; Immunology; Oncology; T cells.
Conflict of interest statement
Figures



Update of
-
Rare Subset of T Cells Form Heterotypic Clusters with Circulating Tumor Cells to Foster Cancer Metastasis.bioRxiv [Preprint]. 2025 Apr 3:2025.04.01.646421. doi: 10.1101/2025.04.01.646421. bioRxiv. 2025. Update in: J Clin Invest. 2025 Sep 16;135(18):e193521. doi: 10.1172/JCI193521. PMID: 40236049 Free PMC article. Updated. Preprint.
References
-
- Ashworth T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J. 1869;14:146–147.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

