CARI Guidelines Commentary on the KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases
- PMID: 40955731
- PMCID: PMC12439324
- DOI: 10.1111/nep.70119
CARI Guidelines Commentary on the KDIGO Clinical Practice Guideline for the Management of Glomerular Diseases
Abstract
Aim: The KDIGO 2021 Glomerular Disease Guidelines provide updated recommendations on the management of glomerular diseases (GD), with substantial advances made in diagnosis, treatment, and improvement of outcomes for people with GD.
Methods: The Caring for Australians and New Zealanders with kidney Impairment (CARI) Guidelines commentary contextualises the updated guidelines for the Australian and New Zealand setting.
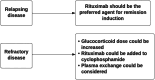
Results: Kidney biopsy remains central to diagnosis, with validated scoring systems available. However, genetic testing for suspected monogenic kidney disease is now accessible in Australia, enabling earlier diagnosis and management, particularly in situations where a kidney biopsy is considered high risk or contraindicated. The guideline emphasises timed urine collections for protein excretion over spot tests and we suggest the use of the CKiD u25 eGFR equation for people under 25. For IgA nephropathy (IgAN) and IgA vasculitis (IgAV), emerging therapies such as targeted-release budesonide and sparsentan demonstrate promise but await approval and public subsidy in our region. For membranous nephropathy, the guideline highlights the differences in adult and paediatric management. In nephrotic syndrome, tacrolimus is used as first-line therapy and rituximab as a second-line agent for steroid-dependent or frequently relapsing disease. Minimal change disease recommendations include glucocorticoid tapering after remission, while focal segmental glomerulosclerosis incorporates genetic classifications and advocates for next-generation sequencing.
Conclusion: Our commentary underscores the need for increased participation in clinical trials to validate regional applicability and improve long-term outcomes for people with GD in Australia and New Zealand. Clinical trials of new medications have led to more treatment options that are awaiting approval.
Keywords: IgA nephropathy; antineutrophil cytoplasmic antibody (ANCA); glomerulonephritis (GN); guidelines; lupus nephritis; nephrotic syndrome.
© 2025 The Author(s). Nephrology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Nephrology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group , “KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases,” Kidney International 100, no. 4S (2021): S1–S276. - PubMed
-
- Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group , “KDIGO 2024 Clinical Practice Guideline for the Management of Antineutrophil Cytoplasmic Antibody (ANCA)‐Associated Vasculitis,” Kidney International 105, no. 3s (2024): S71–S116. - PubMed
-
- Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group , “KDIGO 2024 Clinical Practice Guideline for the Management of Lupus Nephritis,” Kidney International 105, no. 1s (2024): S1–S69. - PubMed
-
- Floege J., Gibson K. L., Vivarelli M., Liew A., Radhakrishnan J., and Rovin B. H., “KDIGO 2025 Clinical Practice Guideline for the Management of Nephrotic Syndrome in Children,” Kidney International 107, no. 5s (2025): S241–S289. - PubMed
-
- Bajema I. M., Wilhelmus S., Alpers C. E., et al., “Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices,” Kidney International 93, no. 4 (2018): 789–796. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous