Combination of Treadmill Training and Inosine Enhance Nerve Regeneration and Functional Recovery After Mice Sciatic Nerve Transection
- PMID: 40956018
- PMCID: PMC12439521
- DOI: 10.1002/jnr.70080
Combination of Treadmill Training and Inosine Enhance Nerve Regeneration and Functional Recovery After Mice Sciatic Nerve Transection
Abstract
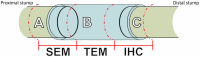
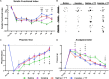
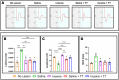
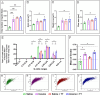
Peripheral nerve injuries are a major cause of disability, leading to significant sensorimotor impairment and functional loss. These injuries can result from traumatic or non-traumatic events, with severe cases posing therapeutic challenges. Neurorrhaphy is the gold standard for treating injuries where the gap between nerve stumps is less than 3 cm, while autografting is used for larger gaps. Despite various therapeutic approaches aimed at enhancing peripheral nerve regeneration, restoring pre-injury function remains difficult in clinical practice, prompting the exploration of experimental therapies. This study examined the effects of treadmill training and inosine treatment on sciatic nerve regeneration after transection in mice. Male C57/Bl6 mice (8-12 weeks) underwent sciatic nerve transection, with the proximal and distal stumps sutured to a polylactic acid tubular graft, creating a 3 mm gap. The mice were treated with saline or inosine (70 mg/mL) for 1 week, followed by treadmill exercise starting in the second week. The exercise protocol involved treadmill speeds of 6-12 m/min, three times per week for 10 min, continuing for 8 weeks. Functional recovery was assessed weekly using the Sciatic Functional Index, pinprick test, and Von Frey electronic analgesiometer. At the end of the study, electrophysiological tests and morphologic analysis were performed. The results showed that the combination of inosine with treadmill training significantly accelerated functional recovery and nerve regeneration, suggesting that this combined approach may offer a promising alternative for improving recovery outcomes in cases of peripheral nerve injury.
Keywords: inosine; nerve guidance conduit; nerve regeneration; peripheral nerve injury; treadmill training.
© 2025 The Author(s). Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

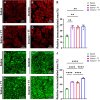
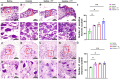
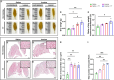
References
-
- Albus, C. A. , Rishal I., and Fainzilber M.. 2013. “Cell Length Sensing for Neuronal Growth Control.” Trends in Cell Biology 23, no. 7: 305–310. - PubMed
-
- Awad, A. S. , Huang L., Ye H., et al. 2006. “Adenosine A2A Receptor Activation Attenuates Inflammation and Injury in Diabetic Nephropathy.” American Journal of Physiology. Renal Physiology 290, no. 4: F828–F837. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

