Positive correlation between structural disorder of the HIV-1 Gag N-terminal segment and progeny virus particle formation
- PMID: 40956082
- PMCID: PMC12548442
- DOI: 10.1128/jvi.00887-25
Positive correlation between structural disorder of the HIV-1 Gag N-terminal segment and progeny virus particle formation
Abstract
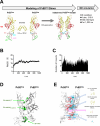
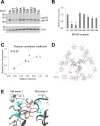
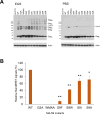
A class of unstructured peptide segments termed disordered regions plays a crucial role in the regulation of protein structure and function. Although the N-terminal region of the matrix (MA) domain of the HIV-1 Gag precursor protein is composed of an unstructured peptide, the mechanisms underlying the regulation of the unstructured state relating to control of viral phenotypes remain unclarified. We examined, in association, the structural, evolutionary, and biological roles of the MA N-terminal region via mutagenesis. Molecular dynamics simulation of a full-length Gag dimer model suggested that an amino acid residue at position 9 in the MA N-terminal region (MA-9) participates in the Gag dimerization. Information entropy analysis indicated that the MA-9 residue is variable in nature, but the hydrophobic amino acid substitution is evolutionarily maladaptive. Disordered region prediction study suggested that single hydrophobic amino acid substitutions at MA-9 reduce the disordered state of the MA N-terminal region. Consistently, NMR analysis indicated that such substitution reduces motional dynamics of the MA N-terminal region and alters the conformation of the MA domain. A site-directed mutagenesis study showed that hydrophobic amino acid substitutions at the MA-9 residue impair, to different degrees, the elementary and overall processes of virus particle formation in the cells. Importantly, the level of the virus particle formation was positively correlated with the level of disorder of the MA N-terminal region. These results indicate that the maintenance of structural disorder and dynamics of the Gag N-terminal segment is regulated by the MA-9 residue and critical for maintaining the optimal production of HIV-1 particles.IMPORTANCEA class of unstructured peptide segments termed disordered regions plays crucial roles in the regulation of protein structure and function. Although HIV-1 Gag precursor protein has multiple disordered elements, molecular mechanisms underlying regulation of unstructured state and viral phenotypes largely remain elusive. In this study, by analyzing in association the structural, evolutionary, and virological roles of the disordered N-terminal region of the HIV-1 Gag protein, we show that an amino acid residue at position 9 of the Gag is able to modulate the N-terminal disordered state, and the level of disorder of the Gag N-terminal region is positively correlated with the level of virus particle formation. Our findings gain new insights into molecular mechanisms of regulation of Gag structure and highlight the importance of a previously unappreciated survival strategy of HIV-1-namely, preservation of the Gag N-terminal disorder.
Keywords: Gag; HIV-1; structural disorder; virus particle production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Dunker AK, Fuxreiter M, Gough J, Gsponer J, Jones DT, Kim PM, Kriwacki RW, Oldfield CJ, Pappu RV, Tompa P, Uversky VN, Wright PE, Babu MM. 2014. Classification of intrinsically disordered regions and proteins. Chem Rev 114:6589–6631. doi: 10.1021/cr400525m - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

