Lactate Activates the HCAR1/β-Arrestin2/PP2A Signaling Axis to Mediate STAT1/2 Dephosphorylation and Drive Osteosarcoma Progression
- PMID: 40956299
- PMCID: PMC12677626
- DOI: 10.1002/advs.202506214
Lactate Activates the HCAR1/β-Arrestin2/PP2A Signaling Axis to Mediate STAT1/2 Dephosphorylation and Drive Osteosarcoma Progression
Abstract
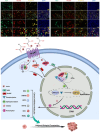
The "Warburg effect", a hallmark of Osteosarcoma(OS), results in lactate accumulation due to aerobic glycolysis. The role and underlying mechanisms of lactate in OS are not well understood. Herein, the lactate-activated hydroxycarboxylate receptor 1(HCAR1) is found to promote OS progression via inhibiting the transcription of anti-oncogene downstream of STAT1/2. The phosphorylation level of STAT1/2 holds considerable significance for transcriptional activity. In this study, protein phosphatase 2A(PP2A) is identified as the tyrosine phosphatase of STAT1/2. Lactate-activated HCAR1, facilitating PP2A interaction with phosphorylated STAT1/2 via β-Arrestin 2, resulting in STAT1/2 dephosphorylation, a key process linked to the aggressive behavior of OS. Using PP2A inhibitor Endothall can abolish the dephosphorylation effect of HCAR1 on STAT1/2, inhibit cancer cell proliferation, migration, and cell cycle, and promote apoptosis. Moreover, the combination of Endothall and Cisplatin is high synergistic in treating OS. In conclusion, the study elucidates the pro-oncogenic role of lactate-activated HCAR1 in OS.
Keywords: HCAR1; STAT1/2; lactate; osteosarcoma; β‐Arrestin 2.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
- 82172779/National Natural Science Foundation of China
- 82372609/National Natural Science Foundation of China
- 82573785/National Natural Science Foundation of China
- 82305057/National Natural Science Foundation of China
- 81874180/National Natural Science Foundation of China
- 82373297/National Natural Science Foundation of China
- 82160592/National Natural Science Foundation of China
- 82302745/National Natural Science Foundation of China
- 82073266/National Natural Science Foundation of China
- 82273471/National Natural Science Foundation of China
- XPT82305057/The Matching Foundation of National Natural Science Foundation of China
- 23QA1411700/Shanghai Rising-Star Program
- ZYQ20025/Nanjing Young Talents Project of Traditional Chinese Medicine
- ZYQN202204/Nanjing Young Talents Project of Traditional Chinese Medicine
- 2024GXNSFAA010168/General program of the Natural Science Foundation of Guangxi
LinkOut - more resources
Medical
Research Materials
Miscellaneous
