Estimands for Clinical Effectiveness of Risk-Reducing Early Salpingectomy in Women With High Risk of Ovarian Cancer
- PMID: 40956577
- PMCID: PMC12441877
- DOI: 10.1001/jamanetworkopen.2025.32195
Estimands for Clinical Effectiveness of Risk-Reducing Early Salpingectomy in Women With High Risk of Ovarian Cancer
Erratum in
-
Errors in Abstract and Methods.JAMA Netw Open. 2025 Oct 1;8(10):e2542056. doi: 10.1001/jamanetworkopen.2025.42056. JAMA Netw Open. 2025. PMID: 41060658 Free PMC article. No abstract available.
Abstract
Importance: Risk-reducing early-salpingectomy (RRES) and delayed oophorectomy (DO) is a novel 2-stage alternative prevention strategy to risk-reducing salpingo-oophorectomy (RRSO) that avoids detrimental consequences of premature menopause. However, direct data on the clinical effectiveness for ovarian cancer (OC) risk reduction are lacking.
Objective: To explore how to define clinical effectiveness from prospective cohort studies using the estimand framework and sample size requirements.
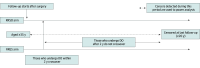
Design, setting, and participants: In this comparative effectiveness research study, estimand and analysis options were considered to evaluate the clinical effectiveness of RRES with DO by extending the UK PROTECTOR cohort study, a multicenter, prospective, observational, national cohort study (N = 1250 recruited from January 1, 2019, to December 31, 2024) evaluating RRES and DO for OC surgical prevention. Participants were premenopausal women 30 years or older at increased OC risk due to BRCA1/BRCA2 pathogenic variants. Participants could choose RRES, RRSO, or no surgery at entry. Sample size requirements used initial data (eg, age and BRCA1/2 distribution) from PROTECTOR (analysis undertaken from January 1, 2024, to December 31, 2025).
Main outcomes and measures: Incidence of OC after (not at) RRES and before or at DO in women with normal histologic analysis findings at surgery. The proportion of cancers prevented was estimated as the completement of the observed (O) to expected (E; assuming no preventive effect of surgery) number of cancers detected (1 - O/E).
Results: Initial data were obtained from 889 women in PROTECTOR (overall mean [SD] age, 39 [5] years), with 255 (28.7%) choosing RRSO (mean [SD] age, 42 [4] years), 405 (45.5%) choosing RRES (mean [SD] age, 38 [4] years), and 229 (25.7%) choosing no surgery (mean [SD], 38 [5] years). The preferred estimand outcome was OC incidence after surgery (RRES or RRSO) with a "while on intervention" strategy to account for intercurrent events. The primary target measure was the proportion of cancers prevented for RRES vs no surgery with superiority testing. The secondary target measure was noninferiority of RRES vs RRSO. An estimated 1150 RRES participants with 8 to 10 years of follow-up would provide approximately 92% power to show that 20% or more of cancers are prevented using a 1-sample binomial test of the O:E risk (external reference) at the 5% level under a range of assumptions and at least the same power for a noninferiority margin for the proportion of cancers prevented by RRES of those prevented by RRSO. Estimands based on incidence ratios had an infeasible sample size.
Conclusions and relevance: In this comparative effectiveness study of UK BRCA carriers, the estimand differed from other ongoing clinical effectiveness studies of RRES and DO. Advantages include direct use of expected risk at baseline (unknown at design stage), easier interpretation across cohorts than absolute risk differences, and providing a feasible recruitment target for PROTECTOR to evaluate clinical effectiveness.
Conflict of interest statement
Figures



References
-
- International Agency for Research on Cancer . Cancer Tomorrow: A Tool That Predicts the Future Cancer Incidence and Mortality Burden Worldwide From the Current Estimates in 2018 Up Until 2040. IARC; 2018.
-
- Menon U, Gentry-Maharaj A, Burnell M, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2021;397(10290):2182-2193. doi: 10.1016/S0140-6736(21)00731-5 - DOI - PMC - PubMed
-
- National Institute for Health and Care Excellence . Ovarian Cancer: Identifying and Managing Familial and Genetic Risk. NICE guideline NG241. Accessed February 22, 2025. https://www.nice.org.uk/guidance/ng241
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

