Abrogation of FGFR signaling blocks β-catenin-induced adrenocortical hyperplasia and aldosterone production
- PMID: 40956622
- PMCID: PMC12643481
- DOI: 10.1172/jci.insight.184863
Abrogation of FGFR signaling blocks β-catenin-induced adrenocortical hyperplasia and aldosterone production
Abstract
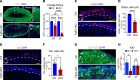
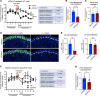
Fibroblast growth factor receptors (FGFRs) are tyrosine kinase receptors critical for organogenesis and tissue maintenance, including in the adrenal gland. Here we delineate the role of FGFR2 in the morphogenesis, maintenance, and function of the adrenal cortex with a focus on the zona glomerulosa (zG). zG-specific Fgfr2 deletion (Fgfr2-cKO) resulted in impaired zG cell identity, proliferation, and transdifferentiation into zona fasciculata (zF) cells during postnatal development. In adult mice, induced deletion of Fgfr2 led to loss of mature zG cell identity, highlighting the importance of FGFR2 for the maintenance of a differentiated zG state. Strikingly, Fgfr2-cKO was sufficient to fully abrogate β-catenin-induced zG hyperplasia and to reduce aldosterone levels. Finally, short-term treatment with pan-FGFR small molecule inhibitors suppressed aldosterone production in both WT and β-catenin gain-of-function mice. These results demonstrate a critical role for FGFR signaling in adrenal morphogenesis, maintenance, and function and suggest that targeting FGFR signaling may benefit patients with aldosterone excess and/or adrenal hyperplasia.
Keywords: Cardiology; Endocrinology; Homeostasis; Hypertension; Mouse models.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

