Real-world effectiveness and safety of ofatumumab in relapsing-remitting multiple sclerosis: Insights from naïve and switch patients
- PMID: 40957770
- PMCID: PMC12664450
- DOI: 10.1016/j.neurot.2025.e00724
Real-world effectiveness and safety of ofatumumab in relapsing-remitting multiple sclerosis: Insights from naïve and switch patients
Abstract
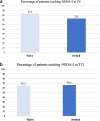
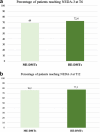
Ofatumumab (OFA), a fully human anti-CD20 monoclonal antibody, has shown promising efficacy in treating relapsing multiple sclerosis (RMS) by depleting B cells and reducing disease activity. This real-world, prospective, multicenter study evaluated the effectiveness and safety of OFA in treatment-naïve patients and those transitioning from other disease-modifying therapies (DMTs), including natalizumab (NTZ). RRMS patients initiating OFA at seven MS centers in Sicily and treated for at least 12 months were analyzed. Outcomes included annualized relapse rates (ARR), Expanded Disability Status Scale (EDSS), and the percentage of patients free from relapse, MRI activity, and confirmed EDSS worsening (CEW). Of 213 patients, 66 (30.9 %) were naïve and 147 (69.1 %) were switchers. At 12 months, both groups showed comparable CEW-free (93.9 % vs. 93.8 %), relapse-free (92.4 % vs. 93.2 %), and MRI activity-free (84.8 % vs. 85.0 %) proportions. Within the high-efficacy group, NTZ-switchers showed significantly better MRI outcomes than those switching from other agents, while CEW-free and relapse-free rates remained similar. OFA was well tolerated with no serious adverse events. Predictors of non-response included high baseline MRI activity, disease duration >10 years, and prior NTZ and non-NTZ high-efficacy DMTs. These findings support OFA as a safe and effective option for RRMS across patient subtypes.
Keywords: Multiple sclerosis; Ofatumumab; Switch.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Clara Grazia Chisari reports a relationship with Sanofi Genzime that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Li R., Patterson K.R., Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696–707. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

