Mechanical Stimulation Induces Yap Mediated OCTN2 Transcription to Enhance Carnitine Metabolism in Sarcopenia
- PMID: 40958655
- PMCID: PMC12441309
- DOI: 10.1002/jcsm.70052
Mechanical Stimulation Induces Yap Mediated OCTN2 Transcription to Enhance Carnitine Metabolism in Sarcopenia
Abstract
Background: Sarcopenia is a systemic skeletal muscle disease that seriously affects the health of the aged population. Exercise prevents sarcopenia, but the underlying mechanobiological and metabolic mechanisms need to be further investigated.
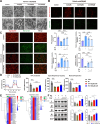
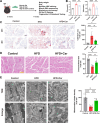
Methods: Carnitine and organic cation transporter 2 (OCTN2) levels were assessed in humans and animals with sarcopenia. Skeletal muscle function and histomorphology were assessed in an animal model. Mitochondrial structure and function were assessed via MitoSox and JC-1 staining, seahorse assays and electron microscopy. Molecular mechanisms were assessed by Western blot analysis, qPCR, a luciferase reporter gene assay, chromatin immunoprecipitation and immunofluorescence in C2C12 myotubular cells.
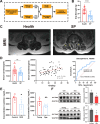
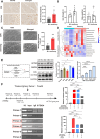
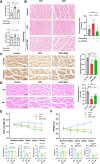
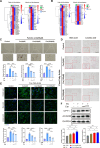
Results: A total of 66 patients were included in the study (Healthy group, % females: 44.74%, mean age: 67.40 ± 8.2, mean BMI: 24.7 ± 3.80 kg/m2; Sarcopenia group, % females: 39.29%, mean age: 71 ± 8.42, mean BMI: 23.1 ± 2.98 kg/m2). Serum carnitine levels decreased in sarcopenia patients (10 868 ± 3466 ng/mL vs. 8469 ± 2360 ng/mL, p < 0.01). Carnitine is an independent protective factor for sarcopenia (OR, 0.757; 95% CI 0.599-0.923, p = 0.0107). Carnitine and OCTN2 levels also decreased in the muscles of mice with dexamethasone-induced muscle atrophy (carnitine: -16.5%, p < 0.05) and aged mice (carnitine: -32.03%, p < 0.01). Suppressed expression of OCTN2 led to a decrease in muscle carnitine (2983 ± 466.3 ng/mL vs. 2517 ± 355.3 ng/mL, p < 0.05), as well as muscle atrophy in mice. Swimming exercise enhanced mice carnitine-dependent fatty acid oxidation and increased OCTN2 expression (OCTN2: +8.4%, p < 0.05). Knockdown of OCTN2 partially reduced this effect during swimming. Cellular experiments revealed that mechanical stimulation upregulated OCTN2 expression. OCTN2 knockdown impaired myotube formation and led to the disruption of the cellular mitochondrial structure. Further mechanistic studies showed that mechanical forces enhanced OCTN2 transcription and regulated carnitine metabolic homeostasis through the Yap/Tead4 pathway. Yap agonist XMU alleviated dexamethasone-induced muscle atrophy (grip: +13%, p < 0.05; cross-sectional area of the gastrocnemius muscle: +8%, p < 0.05). In a high-fat diet mouse model and in cellular experiments, carnitine supplement improved mitochondrial structure and alleviated mitochondrial dysfunction by reducing excessive lipid accumulation and thus altered myocyte fate.
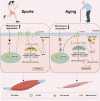
Conclusion: Swimming and carnitine supplementation alleviated sarcopenia. The mechanism was closely related to the enhancement of OCTN2 expression after Yap activation and the enhancement of carnitine-mediated lipid metabolism. These findings reveal exercise regulates skeletal muscle by coupling mechanics and metabolism synergetically. We provide a new therapeutic strategy for sarcopenia.
Keywords: OCTN2; Yap/Tead4; carnitine; fatty acids; lipid deposition; mechanical force; mitochondrial dysfunction; muscular atrophy.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

