Engineering ultrapotent trivalent anticoagulants through hybridisation of salivary peptides from multiple haematophagous organisms
- PMID: 40959396
- PMCID: PMC12434495
- DOI: 10.1039/d5sc04734j
Engineering ultrapotent trivalent anticoagulants through hybridisation of salivary peptides from multiple haematophagous organisms
Abstract
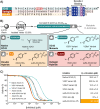
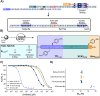
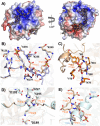
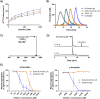
Haematophagous organisms are a rich source of salivary anticoagulant polypeptides that exert their activity by blocking the catalytic site and one of two positively charged exosites on the host protease thrombin. Here, we describe a molecular engineering approach to hybridise post-translationally sulfated polypeptides from different blood-feeding organisms to enhance anticoagulant activity. This led to the discovery of a triply sulfated hybrid anticoagulant, XChimera, possessing fragments from flea, leech, and fly salivary polypeptides that exhibits femtomolar inhibitory activity against thrombin. The crystallographic structure of a complex of XChimera with thrombin shows that it displays a trivalent binding mode in which it simultaneously blocks three functional sites of the protease, the active site and exosites I and II. This trivalent chimera exhibited ultrapotent anticoagulant activity in a suite of in vitro clotting assays and was also shown to possess potent in vivo antithrombotic activity in a murine model of thrombosis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
During the performance of all research studies I. A. and S. M. S. were employed by the Heart Research Institute, and affiliated with the University of Sydney, receiving salaries funded by academic grants. These authors are currently employed by ThromBio Pty Ltd, with the latter employment commencing after initial submission of the manuscript. S. P. J. is the founder and director of ThromBio Pty Ltd.
Figures
References
LinkOut - more resources
Full Text Sources

