The role of extracorporeal CO2 removal from pathophysiology to clinical applications with focus on potential combination with RRT: an expert opinion document
- PMID: 40959434
- PMCID: PMC12433871
- DOI: 10.3389/fmed.2025.1651213
The role of extracorporeal CO2 removal from pathophysiology to clinical applications with focus on potential combination with RRT: an expert opinion document
Abstract
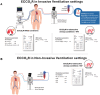
Technological advancements have facilitated the application of extracorporeal-carbon-dioxide removal (ECCO2R) in managing acute respiratory-failure (ARF), including both hypoxemic and hypercapnic forms. A non-systematic literature review (PubMed, Medline, Embase, Google Scholar; January 2000-November 2024) identified randomized-controlled-trials (RCTs) and real-world evidence (RWE) on ECCO2R, alone or combined with continuous renal replacement therapy (CRRT). A multidisciplinary panel of intensivists, anesthesiologists, and nephrologists from Italy, Portugal, and Spain assessed clinical integration of ECCO2R. Key considerations included identifying ideal candidates, such as patients with acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease (COPD), asthma exacerbations, alongside initiation timing and discontinuation criteria. For ARDS, recommended initiation thresholds included driving pressure ≥15 cm H2O, plateau pressure ≥28 cm H2O, pH < 7.28, and respiratory-rate >25 breaths/min. In COPD or asthma exacerbations at risk of non-invasive ventilation (NIV) failure, triggers included pH ≤ 7.25, RR ≥ 30 breaths/min, Intrinsic-PEEP ≥ 5 cm H2O, signs of respiratory fatigue, paradoxical abdominal motion, and severe distress. Absolute contraindications were uncontrolled bleeding, refractory hemodynamic instability, or lack of vascular access. Relative contraindications included moderate coagulopathy and limited access. The panel concluded ECCO2R may support selected adults with ARDS or obstructive lung disease, though further RCTs and high-quality prospective studies are needed to guide practice.
Keywords: acute distress respiratory syndrome; asthma; chronic obstructive pulmonary disease; continuous renal replacement therapy; extracorporeal CO2 removal; mechanical ventilation.
Copyright © 2025 Parrilla-Gómez, Castelli, Colombo, do Vale-Fernandes, Nalesso, Pestaña-Lagunas, Suarez-Sipmann and Terragni.
Conflict of interest statement
FP-G has received remuneration for clinical sessions delivered in the context of ECCO2R from Baxter and Cardiolink Group. DP-L has received honoraria as speaker from Braun, Baxter, and Maquet. FS-S has received payments for clinical sessions delivered in the context of ECCO2R from Baxter. He is member of the medical advisory boards of Getinge and Hamilton and have received research grants from Timpel and Air Liquide. PT has received payments for lectures from Baxter and Braun. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

