Photoswitches beyond azobenzene: a beginner's guide
- PMID: 40959513
- PMCID: PMC12434931
- DOI: 10.3762/bjoc.21.143
Photoswitches beyond azobenzene: a beginner's guide
Abstract
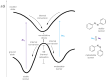
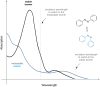
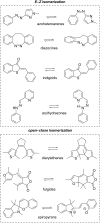
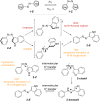
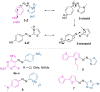
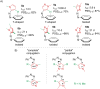
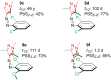
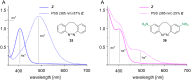
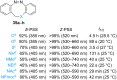
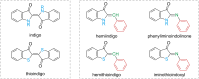
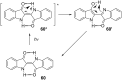
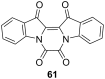
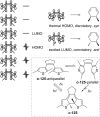
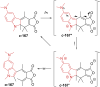
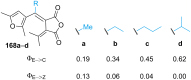
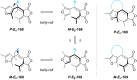
Approaching the vast, colourful world of photoswitches from a different field of study or as an undergraduate student may be overwhelming: azobenzene is undoubtedly the most famous due to its easy synthesis and the extensively studied properties. However, there are several photoswitch classes beyond azobenzene with interesting properties that can be tailored to meet one's needs. In this tutorial review, we aim to explain the important terminology and discuss the synthesis, switching mechanisms, and properties of seven interesting photoswitch classes, namely azoheteroarenes, diazocines, indigoid photoswitches, arylhydrazones, diarylethenes, fulgides, and spiropyrans.
Keywords: photoswitch properties; photoswitches; switching mechanisms; synthesis of photoswitches; tutorial review.
Copyright © 2025, Marcon et al.
Figures
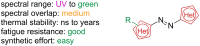
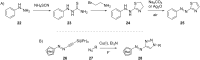
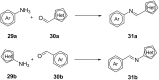
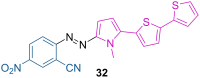
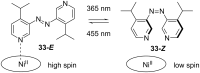
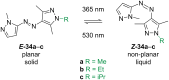

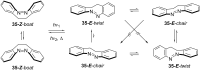
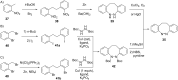
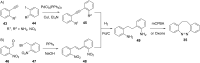
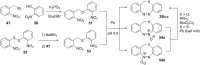
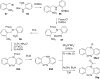
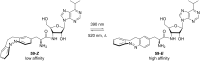


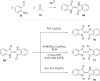
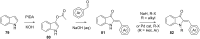
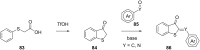
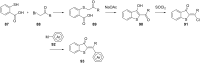

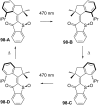
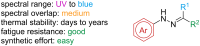
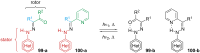
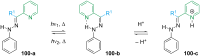

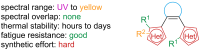
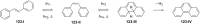
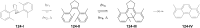

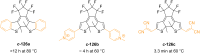
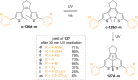

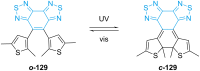
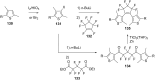
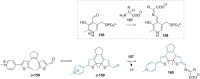
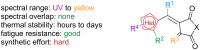
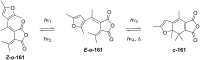
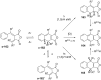
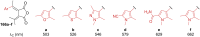

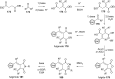
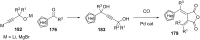
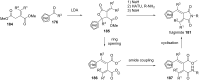

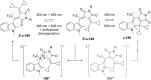

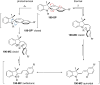
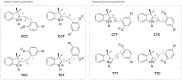
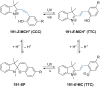
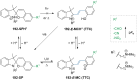

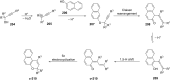
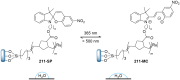

References
Publication types
LinkOut - more resources
Full Text Sources
