Septin4 Regulates Cardiac Fibrosis After Pressure Overload
- PMID: 40960950
- PMCID: PMC12466173
- DOI: 10.1161/CIRCRESAHA.125.326758
Septin4 Regulates Cardiac Fibrosis After Pressure Overload
Abstract
Background: In response to cardiac injury the mammalian heart undergoes ventricular remodeling to maintain cardiac function. These changes are initially considered compensatory, but eventually lead to increased cardiomyocyte apoptosis, reduced cardiac function and fibrosis which are important contributors to the development of heart failure. The small GTPase Sept4 (Septin4) has previously been implicated in the regulation of regeneration and apoptosis in several organs. However, the role of Sept4 in regulating the response of the heart to stress is unknown.
Methods: Ten-week-old wild-type (WT) and Sept4 knockout mice were subjected to transverse aortic constriction to induce cardiac injury. Genotype-dependent differences were investigated at baseline and at 1- and 4-week postinjury time points. To definitively establish the fibroblast-specific cardioprotective effects of Sept4, we generated a fibroblast-specific Sept4 conditional knockout model.
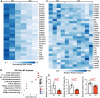
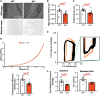
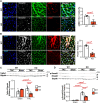
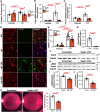
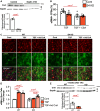
Results: Under homeostatic conditions Sept4 knockout mice showed normal cardiac function comparable with WT controls. In response to transverse aortic constriction, WT mice developed reduced cardiac function and heart failure, accompanied by an increase in cardiomyocyte apoptosis. In contrast, knockout mice were protected against injury with maintenance of normal cardiac function and reduced levels of cardiomyocyte apoptosis. Both at baseline and after transverse aortic constriction, knockout hearts exhibited decreased levels of cardiac extracellular matrix deposition and fibrosis compared with WT controls. In support of these data, the level of myofibroblast activation was lower after injury in knockout mice. Furthermore, the knockout group showed higher levels of cardiac compliance and improved diastolic function compared with WT controls. Mechanistically, we identified reduced fibrosis development due to alterations in calcineurin-dependent signaling in fibroblasts. These results were further verified in fibroblast-specific conditional Sept4 knockout mice subjected to cardiac pressure overload.
Conclusions: We identified Sept4 as an important regulator of extracellular matrix remodeling in the heart. Sept4 controls the conversion of fibroblast to myofibroblast through calcineurin-dependent mechanisms.
Keywords: cardiovascular disease; constriction; fibrosis; heart failure; myofibroblast.
Conflict of interest statement
None.
Figures



References
-
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 - PMC - PubMed
-
- Gogiraju R, Xu X, Bochenek ML, Steinbrecher JH, Lehnart SE, Wenzel P, Kessel M, Zeisberg EM, Dobbelstein M, Schafer K. Endothelial p53 deletion improves angiogenesis and prevents cardiac fibrosis and heart failure induced by pressure overload in mice. J Am Heart Assoc. 2015;4:e001770. doi: 10.1161/JAHA.115.001770 - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

