Capacity and cost benefits of subcutaneous versus intravenous pertuzumab/trastuzumab: The EASE-SC study
- PMID: 40961902
- PMCID: PMC12475515
- DOI: 10.1016/j.breast.2025.104573
Capacity and cost benefits of subcutaneous versus intravenous pertuzumab/trastuzumab: The EASE-SC study
Abstract
Objectives: Subcutaneous administration of pertuzumab and trastuzumab offers a faster alternative to intravenous infusion for patients with HER2-positive breast cancer. However, real-world data on its impact on costs and capacity remain limited. Therefore, this study aimed to compare healthcare resource utilization and costs associated with subcutaneous versus intravenous administration of pertuzumab and trastuzumab from a societal perspective.
Methods: This study was conducted at two Dutch hospitals. Observational data were collected on drug preparation, administration times, and resource use for both formulations. Patient questionnaires assessed societal costs, including travel expenses and productivity losses. Costs were calculated for patient chair time, healthcare professional time, disposables, societal expenses, and drug costs. A nationwide impact analysis estimated potential capacity and productivity gains from switching from intravenous to subcutaneous administration across the Netherlands.
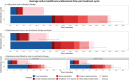
Results: Subcutaneous administration reduced patient chair time compared to intravenous administration, by an average of 106 min (85.5%) for maintenance doses (from 124.3 to 18.1 min) and 287 min (96.0%) for loading doses (from 299.0 to 12.0 min). Active healthcare professional time decreased by 17 min (54.1%) for maintenance doses and 25 min (66.7%) for loading doses. Drug administration costs (excluding drug costs) were lower subcutaneous administration saved approximately €172 per maintenance dose and €403 per loading dose. Nationwide adoption could create capacity for around 22,000 additional treatments annually and save 4.0 full-time equivalent healthcare professionals.
Conclusion: Switching from intravenous to subcutaneous pertuzumab/trastuzumab administration substantially reduces healthcare resource use and may offer cost savings, supporting more efficient delivery of HER2-targeted therapies.
Keywords: Cost analysis; HER2-Positive breast cancer; Healthcare resource utilization; Intravenous administration; Microcosting; Pertuzumab and trastuzumab; Subcutaneous administration.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Loibl S., Gianni L. HER2-positive breast cancer. Lancet. 2017;389(10087):2415–2429. - PubMed
-
- Swain S.M., Miles D., Kim S.B., Im Y.H., Im S.A., Semiglazov V., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. - PubMed
-
- Gianni L., Pienkowski T., Im Y.H., Tseng L.M., Liu M.C., Lluch A., et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. - PubMed
-
- European Medicines Agency (EMA) Summary of product characteristics trastuzumab. https://www.ema.europa.eu/en/documents/product-information/herceptin-epa... Date accessed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

