Reversion of aortic valve cells calcification by activation of Notch signalling via histone acetylation induction
- PMID: 40962819
- PMCID: PMC12443999
- DOI: 10.1038/s41392-025-02411-8
Reversion of aortic valve cells calcification by activation of Notch signalling via histone acetylation induction
Abstract
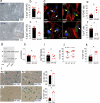
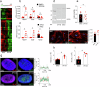
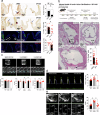
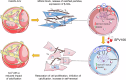
Calcification of the aortic valve is a prevalent cardiovascular pathology in the aging population. Traditionally linked to inflammation, lipid accumulation, and risk conditions, this disease remains poorly understood, and effective treatments to halt its progression are not yet available. We hypothesized that calcification of the human valve interstitial cells (VICs) is associated with cellular senescence and alterations in the epigenetic setup, like in arteries. To verify this hypothesis, we examined the epigenetic marks (DNA methylation; Histones H3/H4 acetylation/methylation), the senescence and the calcification process in human VICs obtained from two distinct pathologic settings of the aortic valve (valve insufficiency and valve stenosis), and employed a mouse model of vascular/valve calcification, based on the administration of Vitamin D. Our findings revealed a link between the senescent phenotype of human VICs and calcification, characterized by increased DNA methylation and changes in histone epigenetic marks. To reverse the senescent/calcific VICs phenotype, we used Pentadecylidenemalonate-1b (SPV106), which activates KAT2B/pCAF histone acetyltransferase. In human VICs, SPV106 restored Histone acetylation marks, modified general chromatin accessibility and upregulated expression of Notch1, a potent inhibitor of valve calcification. The treatment also prevented the accumulation of calcific lesions in an ex vivo model of aortic valve calcification. In vivo treatment with SPV106 reduced calcification of the valve induced by administering Vitamin-D and positively preserved the valve motion compromised by calcification and the overall cardiac function. Based on these results, we propose the treatment with activators of histone acetylates as a viable option to prevent senescence/calcification of aortic VICs via restoration of correct chromatin acetylation, with concrete hopes to retard the progression of valve stenosis, a still largely unmet therapeutic need.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures