A neuronal architecture underlying autonomic dysreflexia
- PMID: 40963010
- PMCID: PMC12571909
- DOI: 10.1038/s41586-025-09487-w
A neuronal architecture underlying autonomic dysreflexia
Abstract
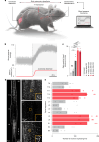
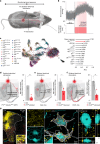
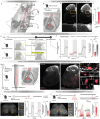
Autonomic dysreflexia is a life-threatening medical condition characterized by episodes of uncontrolled hypertension that occur in response to sensory stimuli after spinal cord injury (SCI)1. The fragmented understanding of the mechanisms underlying autonomic dysreflexia hampers the development of therapeutic strategies to manage this condition, leaving people with SCI at daily risk of heart attack and stroke2-5. Here we expose the neuronal architecture that develops after SCI and causes autonomic dysreflexia. In parallel, we uncover a competing, yet overlapping neuronal architecture activated by epidural electrical stimulation of the spinal cord that safely regulates blood pressure after SCI. The discovery that these adversarial neuronal architectures converge onto a single neuronal subpopulation provided a blueprint for the design of a mechanism-based intervention that reversed autonomic dysreflexia in mice, rats and humans with SCI. These results establish a path towards essential pivotal device clinical trials that will establish the safety and efficacy of epidural electrical stimulation for the effective treatment of autonomic dysreflexia in people with SCI.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.C., A.A.P., J.W.S., J.B., R.D. and S.L. hold various patents in relation to the present work. G.C., A.A.P. and R.D. are consultants of ONWARD Medical. G.C., A.A.P., J.W.S., J.B. and S.L. are minority shareholders of ONWARD, a company with direct relationships with the presented work. The other authors declare no competing interests.
Figures












References
-
- Phillips, A. A. & Krassioukov, A. V. Contemporary cardiovascular concerns after spinal cord injury: mechanisms, maladaptations, and management. J. Neurotrauma32, 1927–1942 (2015). - PubMed
-
- Wu, J.-C. et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology78, 1051–1057 (2012). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

