Evaluation of deuterated [18F]JHU94620 for imaging cannabinoid type 2 receptors in rodent and monkey brain
- PMID: 40963389
- PMCID: PMC12446274
- DOI: 10.1177/0271678X251371377
Evaluation of deuterated [18F]JHU94620 for imaging cannabinoid type 2 receptors in rodent and monkey brain
Abstract
PET imaging of cannabinoid type-2 receptors (CB2Rs) in the healthy brain remains challenging due to low receptor density and the unavailability of radiotracers with high affinity and selectivity. Because some carbon-deuterium bonds are less susceptible than carbon-proton bonds to enzymatic cleavage, deuteration of [18F]JHU94620 was pursued to potentially slow its metabolism. This study: (1) evaluated the sensitivity of a heavily deuterated version of the agonist [18F]JHU94620 ([18F]JHU94620-d8) to detect brain CB2Rs in healthy Sprague-Dawley rats and monkeys and in a rat model of inflammation; (2) assessed the metabolic stability of [18F]JHU94620 and [18F]JHU94620-d8 in whole blood, plasma, and brain of control (FVB) mice and in the whole blood and plasma of monkeys; and (3) investigated the efflux transporter substrate liability of [18F]JHU94620-d8. Deuteration of [18F]JHU94620 did not significantly affect its uptake in the brains of FVB mice, Sprague-Dawley rats, or monkeys, nor did it affect metabolic stability, except in FVB mice. [18F]JHU94620-d8 was also found to be a moderate substrate for efflux transporters in monkeys but not in mice. The sensitivity of [18F]JHU94620-d8 was inadequate to detect the low density of CB2Rs that are in the agonist-preferring state in the brain.
Keywords: CB2 receptor; PET imaging; PET tracer; brain uptake; metabolic stability.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
![The image presents various graphs and images depicting results from experiments with [18F]JHU94620 and its deprotonated form (d8) on mice and rats. Graphs illustrate the radioactivity uptake in brain and jawbone over time, with separate lines for mandible and brain uptakes in both species. Two molecular structures of the respective compounds are also shown, alongside mean PET images displaying radioactivity distribution in the brain and jawbone of mice and rats.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/aefd/12446274/d90eb463941e/10.1177_0271678X251371377-fig1.gif)
![The image presents three radiochromatography graphs comparing the radioactivity clearance rates of [18F]JHU94620 in both brain tissue and plasma after intravenous administration, with an HPLC reference chromatogram.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/aefd/12446274/03da46c04b16/10.1177_0271678X251371377-fig2.gif)
![Comparison of [18F]JHU94620 with [18F]JHU94620-d8 in monkey brain radioactivity uptake, plasma parent concentration, and composition. Graphs in (a) (b) (c) Stable normal distribution volume time. Peaks in (e) (f) Graphs 30 minutes after tracer injection in monkeys. SUV: standardized uptake value.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/aefd/12446274/47aa4a4e5d6f/10.1177_0271678X251371377-fig3.gif)
![This image presents data from a study on a monkey, showing radioactivity uptake in the whole brain and specific regions under baseline and blocked conditions with [18F]JHU94620-d8. Whole brain uptake, plasma parent concentration, and total distribution volume in regions like pons, caudate, putamen, hippocampus, cerebellum, and cortex are depicted. MRI and PET images are included to visualize brain tissue and radiotracer distribution.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/aefd/12446274/1bdaad050ae0/10.1177_0271678X251371377-fig4.gif)
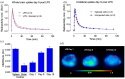
![Whole brain uptake in Monkey and brain regions in Figure 1 : Baseline versus elacridar-treated whole brain and specific brain regions with [18F]JHU94620-d8, showing reduced activity and changes in plasma concentration, with an increase in distribution volume of striatal region.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/aefd/12446274/785478d856d8/10.1177_0271678X251371377-fig6.gif)
References
-
- Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes 2006; 30: S13–S18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

