Surgical management of suspected paraneoplastic myasthenia gravis in rectal cancer during neoadjuvant immunotherapy: a case report
- PMID: 40963855
- PMCID: PMC12436094
- DOI: 10.3389/fonc.2025.1503362
Surgical management of suspected paraneoplastic myasthenia gravis in rectal cancer during neoadjuvant immunotherapy: a case report
Abstract
Colorectal cancer is among the most common malignancies of the gastrointestinal tract. Immune checkpoint inhibitors (ICIs) have become a key component in the treatment of locally advanced rectal cancer (LARC), offering promising therapeutic outcomes. However, ICIs can occasionally cause significant adverse effects. Herein, we report a case of rectal cancer with suspected paraneoplastic myasthenia gravis (pMG) induced by immune checkpoint inhibitors (ICIs). Unfortunately, the patient lacked anti-acetylcholine receptor (AChR)/muscle-specific kinase (MuSK) antibody testing and muscle biopsy, which precluded a definitive diagnosis of pMG. Remarkably, following surgical resection, the patient not only achieved complete tumor eradication but also experienced full remission of myasthenia gravis (MG).
Keywords: immune checkpoint inhibitors; locally advanced rectal cancer; neoadjuvant treatment; paraneoplastic myasthenia gravis; paraneoplastic syndrome.
Copyright © 2025 Xia, Chen, Qiu, Huang, Li, Lin and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
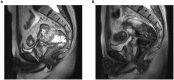
References
-
- Chen G, Jin Y, Guan WL, Zhang RX, Xiao WW, Cai PQ, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-center phase 2 study. Lancet Gastroenterol Hepatol. (2023) 8:422–31. doi: 10.1016/S2468-1253(22)00439-3, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

