A strategy targeting ferroptosis for mitochondrial reprogramming and intervertebral disc degeneration therapy
- PMID: 40963903
- PMCID: PMC12439470
- DOI: 10.7150/thno.117725
A strategy targeting ferroptosis for mitochondrial reprogramming and intervertebral disc degeneration therapy
Abstract
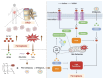
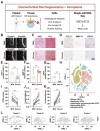
Background: Intervertebral disc degeneration (IVDD) is a leading cause of low back pain, yet current therapies fail to reverse the degenerative process or restore disc function. Ferroptosis, a form of iron-dependent cell death characterized by lipid peroxidation, has been implicated in IVDD progression. Methods: We synthesized Deferoxamine mesylate (DFOM)-loaded cerium oxide nanoparticles (DFOM@CeO2) as a novel ferroptosis-targeting therapeutic. Results: DFOM@CeO2 exhibited dual functionality by scavenging reactive oxygen species (ROS) and chelating excess iron, thereby protecting nucleus pulposus (NP) cells from ferroptosis and extracellular matrix (ECM) degradation. DFOM@CeO2 demonstrated strong antioxidant capacity, effectively reducing iron accumulation and lipid peroxidation, and restoring glutathione peroxidase 4 (GPX4) expression in NP cells. Furthermore, DFOM@CeO2 improved mitochondrial respiratory chain function, reduce mitochondrial ROS production and prevent mitochondrial dysfunction. In a rat model of IVDD, DFOM@CeO2 significantly preserved disc height, reduced ECM degradation, and demonstrated superior therapeutic efficacy compared with DFOM or CeO2 alone. Transcriptome analysis revealed that DFOM@CeO2 modulates key ferroptosis-related genes and promotes mitochondrial reprogramming. Conclusions: These findings highlight DFOM@CeO2 as a promising therapeutic strategy for IVDD, targeting both ferroptosis and mitochondrial dysfunction.
Keywords: ROS scavenging; ceria nanoparticles; deferoxamine mesylate; ferroptosis; intervertebral disc degeneration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2133–61. - PMC - PubMed
-
- Binch ALA, Fitzgerald JC, Growney EA, Barry F. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17:158–75. - PubMed
-
- Genedy HH, Humbert P, Laoulaou B, Le Moal B, Fusellier M, Passirani C. et al. MicroRNA-targeting nanomedicines for the treatment of intervertebral disc degeneration. Adv Drug Deliv Rev. 2024;207:115214. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

