The contrasting regulatory effects of valproic acid on ferroptosis and disulfidptosis in hepatocellular carcinoma
- PMID: 40963919
- PMCID: PMC12439337
- DOI: 10.7150/thno.115661
The contrasting regulatory effects of valproic acid on ferroptosis and disulfidptosis in hepatocellular carcinoma
Abstract
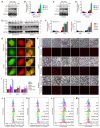
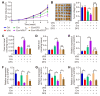
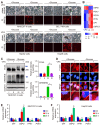
Background: Valproic acid (VPA), a branched short-chain fatty acid, is extensively utilized as both an antiepileptic medication and a mood stabilizer. However, the complete pharmacological functions of VPA on programmed cell death are still not fully understood. In this study, we investigated the role of VPA in modulating ferroptosis and disulfidptosis, which are emerging forms of programmed cell death triggered by lipid peroxidation and disulfide stress respectively. Methods: Herein, the network pharmacology analysis, genome-wide mRNA transcription assay and metabolomics analysis were performed to predict the major pharmacological action and potential targets of VPA. To confirm the hypothesis, pharmacological targeting model and gene knockdown model was created in our work. The pharmacological action of VPA on ferroptosis and disulfidptosis was evaluated respectively. Results: Our findings primarily indicated that the potential targets of VPA were linked to hepatocarcinogenesis and programmed cell death. Additionally, omics data suggested that VPA could significantly influence iron transport and glucose homeostasis. Notably, VPA heightened the susceptibility of hepatocellular carcinoma (HCC) cells to ferroptosis by increasing the labile iron pool, facilitating the accumulation of free iron through enhanced cellular ferritinophagy and reduced ferritin expression. Furthermore, VPA promoted the transcription of glucose-6-phosphate dehydrogenase (G6PD) and impacted glutathione (GSH) metabolism. The activation of the NRF2-G6PD pathway induced by VPA further augmented the production of NADPH and GSH, which subsequently inhibited the formation of disulfide bonds among various cytoskeletal proteins, as well as disulfidptosis in HCC cells. Conclusion: Overall, our results highlight the significant role of VPA in differentially regulating ferroptosis and disulfidptosis in HCC cells, thereby offering a precise avenue for addressing drug-resistant HCC in clinical practice.
Keywords: G6PD; disulfidptosis; ferroptosis; labile iron pool; valproic acid.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Cong L, Dong X, Wang Y, Deng Y, Li B, Dai R. On the role of synthesized hydroxylated chalcones as dual functional amyloid-beta aggregation and ferroptosis inhibitors for potential treatment of alzheimer's disease. Eur J Med Chem. 2019;166:11–21. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

