Dopamine D2 receptor modulating mPFC-BLA circuit contributes to chronic sleep deprivation-induced memory impairment in mice
- PMID: 40963920
- PMCID: PMC12439335
- DOI: 10.7150/thno.114797
Dopamine D2 receptor modulating mPFC-BLA circuit contributes to chronic sleep deprivation-induced memory impairment in mice
Abstract
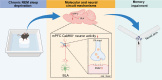
Background: Chronic sleep deprivation (CSD) affects the orchestration of neural networks, leading to cognitive impairment, but the underlying molecular and neural circuitry mechanisms remain unknown. Methods: Mice underwent a two-week CSD regimen, followed by spatial memory assessment using the Y-maze test and EEG gamma oscillation analysis. Dopamine D2 receptor (Drd2) expression in the medial prefrontal cortex (mPFC) was evaluated using transcriptomic and immunofluorescent analysis. The role of Drd2 in CSD-induced memory deficits was examined through local infusion of Drd2 agonists or antagonists into the mPFC. Neural circuit tracing, fiber photometry, and opto-chemogenetic approaches were used to assess Drd2 in the gating of the mPFC-basolateral amygdala (BLA) circuit-mediated memory impairment induced by CSD. Results: CSD disinhibited dopaminergic input to the mPFC and impaired spatial memory in mice. A significant increase in Drd2 expression was found in the layers II/III of the mPFC after CSD. Infusion of Drd2 agonist into the mPFC induced memory deficits in naïve mice, while administration of the Drd2 antagonist reversed memory impairment caused by CSD. Drd2 was found to co-localize with Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα+) neurons in the mPFC that project to the basolateral amygdala (BLA). Activation of CaMKIIα+ neurons restored memory impairment induced by CSD through enhancing mPFC-to-BLA output and reversed memory defects induced by the Drd2 agonist. Conclusion: Our findings demonstrated that excessive Drd2 signaling leads to cognitive impairment following CSD by suppressing mPFC-BLA neurotransmission, suggesting a possible therapeutic value of dopamine D2 receptor antagonists in relieving CSD-induced cognitive decline.
Keywords: Basolateral amygdala; Chronic sleep deprivation; Dopamine D2 receptor; Medial prefrontal cortex; Memory impairment.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Klinzing JG, Niethard N, Born J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. 2019;22:1598–610. - PubMed
-
- Boyce R, Williams S, Adamantidis A. REM sleep and memory. Curr Opin Neurobiol. 2017;44:167–77. - PubMed
-
- Qin H, Fu L, Jian T, Jin W, Liang M, Li J. et al. REM sleep-active hypothalamic neurons may contribute to hippocampal social-memory consolidation. Neuron. 2022;110:4000–14.e6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

