Targeting CB1R Rewires Ca2+-Dependent Mitophagy to Promote Nerve Regeneration
- PMID: 40963929
- PMCID: PMC12439258
- DOI: 10.7150/thno.119712
Targeting CB1R Rewires Ca2+-Dependent Mitophagy to Promote Nerve Regeneration
Abstract
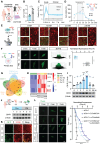
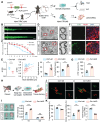
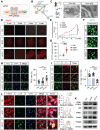
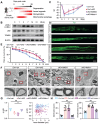
Background: Ion homeostasis is disrupted following nerve injury, and elevated Ca2+ levels have been reported to induce Schwann cell (SC) death. Notably, clinical interventions such as electrical stimulation enhance Ca2+ influx and facilitate nerve regeneration. These findings highlight the need to clarify the precise role of Ca2+ signaling in nerve regeneration. Methods: We assessed extracellular Ca2+ concentrations in both human and murine peripheral nerve tissues after injury. Transcriptomic profiling identified CB1R as a key Ca2+-related gene and in vitro validation was performed with primary cultured SC and nerve explants. A sciatic nerve crush model was established in SC-specific CB1R knockout mice. Mitophagy, cellular metabolic homeostasis, and axonal regeneration were systematically assessed using proteomics, calcium imaging, and in vivo analyses. Additionally, the CB1R antagonist JD5037 was administered in both sciatic and optic nerve injury models to evaluate its translational potential. Results: Peripheral nerve injury (PNI) leads to elevated extracellular Ca2+ levels at the injury site, where a moderate increase (~1.5-fold) favors SC survival. PNI also induces upregulation of CB1R, genetic ablation of CB1R enhances Ca2+ influx, promotes SC survival, and maintains metabolic homeostasis. Mechanistically, CB1R interference upregulates adenine nucleotide translocase 2 (ANT2) expression, promotes mitochondrial permeability transition pore (mPTP) opening and mitochondrial membrane depolarization, thereby activating PINK1/Parkin-mediated mitophagy. This process improves mitochondrial quality and enhances energy metabolic efficiency, ultimately promoting axonal regeneration and functional recovery. Furthermore, systemic administration of the CB1R antagonist JD5037 similarly enhances regeneration of both peripheral and optic nerves in vivo. Conclusion: Moderate extracellular Ca2+ elevation establishes a pro-regenerative microenvironment after nerve injury. Targeting CB1R facilitates Ca2+ influx, enhances mitophagy via the PINK1/Parkin pathway, and promotes nerve regeneration. These findings identify CB1R as a viable therapeutic target and support the translational potential of JD5037 for nerve injury treatment.
Keywords: Ca2+; Schwann cell; cannabinoid receptor 1; mitophagy; nerve regeneration..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

