This is a preprint.
Rapid clearance of bacteria from maternal bloodstream after delivery in pregnancies complicated by preterm pre-labor rupture of the membranes
- PMID: 40964021
- PMCID: PMC12440102
- DOI: 10.21203/rs.3.rs-7359456/v1
Rapid clearance of bacteria from maternal bloodstream after delivery in pregnancies complicated by preterm pre-labor rupture of the membranes
Abstract
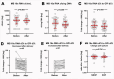
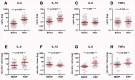
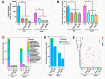
Preterm pre-labor rupture of membranes (PPROM) increases maternal and neonatal sepsis risk, yet its association with maternal microbial translocation and systemic inflammation remains unclear. We investigated whether PPROM is linked to microbial DNA in maternal blood (MB) and inflammatory responses around delivery. In 66 PPROM patients (median GA: 32±1 weeks), MB was collected pre-delivery and within 1 hour postpartum. Fetal membranes (FM) and placental tissues were sampled immediately after delivery. Bacterial load and diversity were analyzed via 16S rDNA qPCR and sequencing. Maternal cytokines were quantified by multiplex immunoassay, and fetal inflammatory exposure (Triple I) assessed using histological chorioamnionitis (HCA), cord blood haptoglobin, and IL-6. Bacterial DNA was detected in maternal blood (MB) pre- and post-delivery, with a significant postpartum decline (p=0.004). FM carried higher bacterial load and biodiversity than placenta (p<0.001), dominated by Mycoplasma spp. Maternal IL-6 and IL-10 levels rose postpartum (p<0.05), particularly in cases with fetal inflammatory exposure. Limited overlap was found between MB and tissue microbial taxa. In conclusion, bacterial DNA is detectable in maternal circulation in patients with PPROM before birth but rapidly clears postpartum alongside a robust cytokine surge, suggesting efficient clearance and dynamic inflammatory changes.
Conflict of interest statement
Conflict of Interest Statement The authors declare no conflicts of interest.
Figures

References
-
- Underwood MA, Gilbert WM, Sherman MP Amniotic fluid: not just fetal urine anymore J Perinatol 2005. May;25(5):341–8. - PubMed
-
- Menon R, Moore JJ Fetal membranes, not a mere appendage of the placenta, but a critical part of the fetal-maternal interface controlling parturition Obstet Gynecol Clin North Am 2020. Mar;47(1):147–162. - PubMed
-
- Seong HS, Lee SE, Kang JH, Romero R, Yoon BH The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor Am J Obstet Gynecol 2008;199(4):375.e1–5.
-
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J The placenta harbors a unique microbiome Sci Transl Med 2014;6(237):237ra65.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

