This is a preprint.
Oropouche virus disrupts neurodevelopment and is vertically transmitted
- PMID: 40964022
- PMCID: PMC12440099
- DOI: 10.21203/rs.3.rs-7512609/v1
Oropouche virus disrupts neurodevelopment and is vertically transmitted
Abstract
Oropouche virus (OROV) historically caused a self-limiting disease, yet recent strains are associated with congenital infection and neurodevelopmental disruption. These cases highlight a need to study OROV as a congenital pathogen and determine the impact of infection on neurodevelopment. Here, we show that OROV is vertically transmitted and induces a microcephaly-like phenotype in human forebrain organoids. We found OROV robustly infects human neural progenitor cells in organoids. In contrast to ZIKV, OROV had a heightened capacity for infection and organoid pathology. We show this increased pathogenesis is partially attributable to OROV antagonism of innate immune signaling. We further demonstrate that OROV is vertically transmitted and infects the fetal tissues in a murine model of congenital infection. Our results demonstrate that OROV can be vertically transmitted and has heightened capacity for neurodevelopmental disruption. These findings underscore need for monitoring OROV as a re-emerging virus capable of inducing microcephaly in infected fetuses.
Conflict of interest statement
COMPETING INTEREST DECLARATION The authors declare no competing interests.
Figures

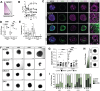
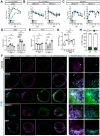
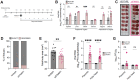
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

