This is a preprint.
Protective immunity against malaria by a nanoparticle CIS43-based junctional vaccine alone or in combination with R21
- PMID: 40964283
- PMCID: PMC12439945
- DOI: 10.1101/2025.09.05.665822
Protective immunity against malaria by a nanoparticle CIS43-based junctional vaccine alone or in combination with R21
Abstract
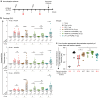
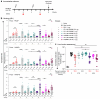
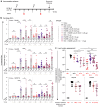
Repetitive display of the major repeats of the Plasmodium falciparum circumsporozoite protein (PfCSP) is the basis for two WHO-recommended vaccines: RTS,S/AS01 and R21/Matrix-M. Recently, however, the CIS43 monoclonal antibody that preferentially targets the junctional region of PfCSP has been shown to be highly protective in humans, highlighting its junctional epitope as a key vaccine target. Here, we develop a vaccine based on the tandem repeats of the junctional epitope displayed on a self-assembling nanoparticle, and compare this CIS43-based junctional vaccine alone or in combination with the benchmark R21 vaccine, using both B cell analysis and monoclonal antibody isolation to define targeting of the immune response. Comparable reduction in liver burden was observed following vaccination with junctional and R21 vaccines at a dose of 1 μg. At a dose of 0.25 μg, a modest reduction of malaria-liver burden with the junctional vaccine was observed compared to R21. Further, combining junctional and R21 vaccines induced modestly enhanced protection compared to either vaccine alone. While the R21 vaccine elicited antibodies primarily against the major repeats, the junctional vaccine elicited antibodies against both junctional and major repeat regions. In vivo-B cell analysis and isolation of monoclonal antibodies confirmed differences in vaccine-induced antibody specificities. Altogether, these data suggest the nanoparticle-formatted tandem-repeated CIS43-junctional vaccine to be a promising approach to broaden immunity against malaria, either as a standalone intervention or in combination with R21.
Keywords: CIS43; PfCSP; R21; antibodies; malaria; self-assembling nanoparticle; structure-based design; vaccine.
Conflict of interest statement
COMPETING INTERESTS STATEMENT The NIH has submitted on behalf of R.A.S., P.D.K., P.T., and B.Z. a US Provisional Patent Application E-237-2024-0-US-01, filed on April 16th, 2025, describing the nanoparticle immunogens and their use. All other authors declare no competing interests. FDB has consultancy relationships with Adimab, Third Rock Ventures, and The EMBO Journal, and founded BliNK Therapeutics.
Figures


References
-
- World malaria report 2024. (2024).
-
- Dame J.B. et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science 225, 593–9 (1984). - PubMed
-
- WHO review of malaria vaccine clinical development. (2025).
-
- Gordon D.M. et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J Infect Dis 171, 1576–85 (1995). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
