This is a preprint.
Febrile temperature enhances Plasmodium falciparum cytoadhesion by disrupting the endothelial glycocalyx
- PMID: 40964286
- PMCID: PMC12440002
- DOI: 10.1101/2025.09.07.674757
Febrile temperature enhances Plasmodium falciparum cytoadhesion by disrupting the endothelial glycocalyx
Abstract
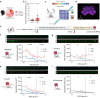
Fever, a universal host defense in infection and inflammation, paradoxically contributes to neurological complications in malaria. While febrile temperatures enhance the expression of parasite virulence proteins that mediate vascular adhesion and disease severity, its effects in the endothelium remain elusive. Here we present a 3D fever-on-a-chip model that recapitulates human brain and lung microvessels under febrile conditions. Short febrile episodes at 40 °C, common in treated cerebral malaria patients, rapidly enhanced iRBC and immune cell binding under flow. Mechanistically, we demonstrated that this phenotype was driven by endothelial glycocalyx shedding, which exposed endothelial receptors EPCR and ICAM-1. Preserving glycocalyx integrity with a broad MMP inhibitor prevented the temperature-induced rise in cytoadhesion. These findings identify fever as a host-specific amplifier of vascular pathology in malaria and highlight endothelial-protective or antipyretic interventions as important strategies to mitigate febrile microvascular pathology.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



References
-
- Lazarus M. et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci 10, 1131–1133 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
